The microbiologist's guide to metaproteomics
- PMID: 40469504
- PMCID: PMC12130581
- DOI: 10.1002/imt2.70031
The microbiologist's guide to metaproteomics
Abstract
Metaproteomics is an emerging approach for studying microbiomes, offering the ability to characterize proteins that underpin microbial functionality within diverse ecosystems. As the primary catalytic and structural components of microbiomes, proteins provide unique insights into the active processes and ecological roles of microbial communities. By integrating metaproteomics with other omics disciplines, researchers can gain a comprehensive understanding of microbial ecology, interactions, and functional dynamics. This review, developed by the Metaproteomics Initiative (www.metaproteomics.org), serves as a practical guide for both microbiome and proteomics researchers, presenting key principles, state-of-the-art methodologies, and analytical workflows essential to metaproteomics. Topics covered include experimental design, sample preparation, mass spectrometry techniques, data analysis strategies, and statistical approaches.
Keywords: bioinformatics; functional dynamics; mass spectrometry; metaproteomics; microbiome.
© 2025 The Author(s). iMeta published by John Wiley & Sons Australia, Ltd on behalf of iMeta Science.
Conflict of interest statement
Daniel Figeys is a Cofounder of MedBiome inc.
Figures

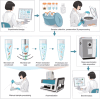

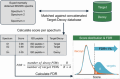

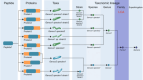

References
-
- Justice, Nicholas B. , Li Zhou, Wang Yingfeng, Spaudling Susan E., Mosier Annika C., Hettich Robert L., and Pan Chongle. 2014. “ (15)N‐ and (2)H Proteomic Stable Isotope Probing Links Nitrogen Flow to Archaeal Heterotrophic Activity.” Environmental Microbiology 16: 3224–3237. 10.1111/1462-2920.12488 - DOI - PubMed
-
- Kleikamp, Hugo B. C. , Grouzdev Denis, Schaasberg Pim, van Valderen Ramon, van der Zwaan Ramon, van de Wijgaart Roel, et al. 2023. “Metaproteomics, Metagenomics and 16S rRNA Sequencing Provide Different Perspectives on the Aerobic Granular Sludge Microbiome.” Water Research 246: 120700. 10.1016/j.watres.2023.120700 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
