Gut microbiota-derived butyrate mediates the anticolitic effect of indigo supplementation through regulating CD4+ T cell differentiation
- PMID: 40469515
- PMCID: PMC12130576
- DOI: 10.1002/imt2.70040
Gut microbiota-derived butyrate mediates the anticolitic effect of indigo supplementation through regulating CD4+ T cell differentiation
Abstract
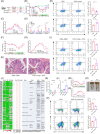
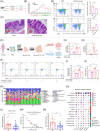
This study explored the effect of plant-derived indigo supplementation on intestinal inflammation using in vivo, in vitro, and clinical sample analyses. Our results showed that indigo decreased mucosal inflammation by regulating CD4+ T cell differentiation in a gut microbiota-dependent manner. Microbes transferred from indigo-treated mice, indigo-induced enrichment of Roseburia intestinalis, and its metabolite butyrate played a role in Th17/Treg immunity similar to that of indigo in intestinal inflammation, which was involved in mTORC1/HIF-1α signal-mediated reprogrammed glucose metabolism. We further showed that patients with ulcerative colitis exhibited significant gut dysbiosis and CD4+ T cell differentiation abnormalities. Our findings provide new insights into the gut-immune axis in ulcerative colitis, offering a novel microbial-based immunotherapy for the treatment of inflammatory bowel disease.
© 2025 The Author(s). iMeta published by John Wiley & Sons Australia, Ltd on behalf of iMeta Science.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
