Establishment of an efficient one-step enzymatic synthesis of cyclic-2,3-diphosphoglycerate
- PMID: 40469723
- PMCID: PMC12136491
- DOI: 10.3389/fmicb.2025.1601972
Establishment of an efficient one-step enzymatic synthesis of cyclic-2,3-diphosphoglycerate
Abstract
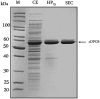
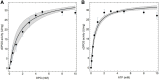
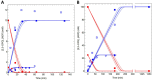
Extremolytes - unique compatible solutes produced by extremophiles - protect biological structures like membranes, proteins, and DNA under extreme conditions, including extremes of temperature and osmotic stress. These compounds hold significant potential for applications in pharmaceuticals, healthcare, cosmetics, and life sciences. However, despite their considerable potential, only a limited number of extremolytes - most notably ectoine and hydroxyectoine - have achieved commercial relevance, primarily due to the absence of efficient production strategies for the majority of other extremolytes. Cyclic 2,3-diphosphoglycerate (cDPG), a unique metabolite found in certain hyperthermophilic methanogenic Archaea, plays a key role in thermoprotection and is synthesized from 2-phosphoglycerate (2PG) through a two-step enzymatic process involving 2-phosphoglycerate kinase (2PGK) and cyclic-2,3-diphosphoglycerate synthetase (cDPGS). In this study, we present the development of an efficient in vitro enzymatic approach for the production of cDPG directly from 2,3-diphosphoglycerate (2,3DPG), leveraging the activity of the cDPGS from Methanothermus fervidus (MfcDPGS). We optimized the heterologous production of MfcDPGS in Escherichia coli by refining codon usage and expression conditions. The purification process was significantly streamlined through an optimized heat precipitation step, coupled with effective stabilization of MfcDPGS for both usage and storage by incorporating KCl, Mg2+, reducing agents and omission of an affinity tag. The recombinant MfcDPGS showed a Vmax of 38.2 U mg-1, with KM values of 1.52 mM for 2,3DPG and 0.55 mM for ATP. The enzyme efficiently catalyzed the complete conversion of 2,3DPG to cDPG. Remarkably, even at a scale of 100 mM, it achieved full conversion of 37.6 mg of 2,3DPG to cDPG within 180 min, using just 0.5 U of recombinant MfcDPGS at 55°C. These results highlight that MfcDPGS can be easily produced, rapidly purified, and sufficiently stabilized while delivering excellent conversion efficiency for cDPG synthesis as value added product. Additionally, a kinetic model for MfcDPGS activity was developed, providing a crucial tool to simulate and scale up cDPG production for industrial applications. This streamlined process offers significant advantages for the scalable synthesis of cDPG, paving the way for further biochemical and industrial applications of this extremolyte.
Keywords: 2-phosphoglycerate kinase; archaea; compatible solutes; cyclic-2,3-diphosphoglycerate synthetase; extremolytes; hyperthermophiles; stress response; thermoprotection.
Copyright © 2025 Stracke, Meyer, De Rose, Ferrandi, Kublanov, Isupov, Harmer, Monti, Littlechild, Müller, Snoep, Bräsen and Siebers.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Cloning, sequencing, and expression of the gene encoding cyclic 2, 3-diphosphoglycerate synthetase, the key enzyme of cyclic 2, 3-diphosphoglycerate metabolism in Methanothermus fervidus.J Bacteriol. 1998 Nov;180(22):5997-6004. doi: 10.1128/JB.180.22.5997-6004.1998. J Bacteriol. 1998. PMID: 9811660 Free PMC article.
-
Biosynthesis of cyclic 2,3-diphosphoglycerate. Isolation and characterization of 2-phosphoglycerate kinase and cyclic 2,3-diphosphoglycerate synthetase from Methanothermus fervidus.FEBS Lett. 1990 Oct 15;272(1-2):94-8. doi: 10.1016/0014-5793(90)80456-s. FEBS Lett. 1990. PMID: 2226838
-
Structural characterization of a novel cyclic 2,3-diphosphoglycerate synthetase involved in extremolyte production in the archaeon Methanothermus fervidus.Front Microbiol. 2023 Nov 16;14:1267570. doi: 10.3389/fmicb.2023.1267570. eCollection 2023. Front Microbiol. 2023. PMID: 38045033 Free PMC article.
-
Extremolytes: Natural compounds from extremophiles for versatile applications.Appl Microbiol Biotechnol. 2006 Oct;72(4):623-34. doi: 10.1007/s00253-006-0553-9. Epub 2006 Sep 7. Appl Microbiol Biotechnol. 2006. PMID: 16957893 Review.
-
Role of the Extremolytes Ectoine and Hydroxyectoine as Stress Protectants and Nutrients: Genetics, Phylogenomics, Biochemistry, and Structural Analysis.Genes (Basel). 2018 Mar 22;9(4):177. doi: 10.3390/genes9040177. Genes (Basel). 2018. PMID: 29565833 Free PMC article. Review.
References
-
- Borges N., Gonçalves L., Rodrigues M., Siopa F., Ventura R., Maycock C., et al. . (2006). Biosynthetic pathways of inositol and glycerol phosphodiesters used by the hyperthermophile Archaeoglobus fulgidus in stress adaptation. J. Bacteriol. 188, 8128–8135. doi: 10.1128/JB.01129-06, PMID: - DOI - PMC - PubMed
-
- Breitung J., Börner G., Scholz S., Linder D., Stetter K. O., Thauer R. K. (1992). Salt dependence, kinetic properties and catalytic mechanism of N-formylmethanofuran:tetrahydromethanopterin formyltransferase from the extreme thermophile Methanopyrus kandleri. Eur. J. Biochem. 210, 971–981. doi: 10.1111/j.1432-1033.1992.tb17502.x, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

