Enhancing lipid production in Nannochloropsis salina via RNAi-mediated downregulation of carbohydrate biosynthesis
- PMID: 40469736
- PMCID: PMC12133928
- DOI: 10.3389/fmicb.2025.1601691
Enhancing lipid production in Nannochloropsis salina via RNAi-mediated downregulation of carbohydrate biosynthesis
Abstract
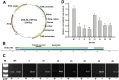
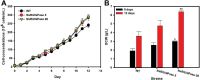
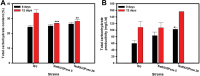
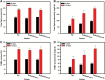
Microalgae are promising platforms for sustainable biofuel production owing to their high photosynthetic efficiency and carbon fixation capacity. Nannochloropsis salina is particularly valued for its robust growth and lipid accumulation. However, redirecting carbon flux from carbohydrate to lipid biosynthesis remains a key challenge in microalgal metabolic engineering. In this study, RNA interference (RNAi) was employed to downregulate uridine diphosphate-glucose pyrophosphorylase (UGPase), a central enzyme in chrysolaminarin biosynthesis. After confirming the presence of core RNAi machinery (Argonaute, Dicer, and RDR) in N. salina, an RNAi construct targeting UGPase was introduced. Two transformants, NsRiUGPase 5 and NsRiUGPase 26, were selected through McrBC-PCR and qRT-PCR screening based on reduced methylation-sensitive PCR band intensity and UGPase transcript levels. These RNAi mutants exhibited significantly enhanced growth compared to wild-type. On day 12, dry cell weight (DCW) reached 4.77 g/L in NsRiUGPase 5 and 6.37 g/L in NsRiUGPase 26, representing 32.4% and 76.9% increases, respectively, compared to WT (3.60 g/L). Despite similar lipid contents per biomass, lipid productivity was markedly improved. On day 12, NsRiUGPase 26 achieved 196.3 mg/L/day, a 71.0% increase over WT (114.8 mg/L/day). Fatty acid methyl ester (FAME) analysis showed no significant difference in lipid composition among strains, indicating that UGPase knockdown did not affect lipid quality. These results demonstrate that RNAi-mediated suppression of UGPase successfully redirected carbon flux away from carbohydrate storage toward growth, thereby enhancing overall lipid productivity. This study provides new insights into carbon partitioning in N. salina and underscores RNAi as a powerful tool for microalgal biofuel optimization.
Keywords: Nannochloropsis salina; RNAi; UGPase; carbohydrate; lipid; microalgae.
Copyright © 2025 Koh, Park, Park, Kim and Kang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Babu S. S., Gondi R., Vincent G. S., Johnsamuel G. C., Jeyakumar R. B. (2022). Microalgae biomass and lipids as feedstock for biofuels: Sustainable biotechnology strategies. Sustainability 14:15070. 10.3390/su142215070 - DOI
-
- Bartley M. L., Boeing W. J., Corcoran A. A., Holguin F. O., Schaub T. (2013). Effects of salinity on growth and lipid accumulation of biofuel microalga Nannochloropsis salina and invading organisms. Biomass Bioenergy 54 83–88. 10.1016/j.biombioe.2013.03.026 - DOI
LinkOut - more resources
Full Text Sources

