Achieving highly efficient carbon radical-mediated cross-coupling reaction in a confined radical microenvironment within a metal-organic framework
- PMID: 40469759
- PMCID: PMC12130900
- DOI: 10.1039/d5sc01242b
Achieving highly efficient carbon radical-mediated cross-coupling reaction in a confined radical microenvironment within a metal-organic framework
Abstract
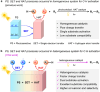
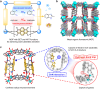
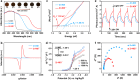
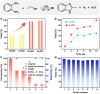
It has been well-demonstrated that the combination of photosensitive (PS), hydrogen atom transfer (HAT) and single electron transfer (SET) processes can achieve efficient radical-mediated organic synthesis, but such reaction systems are usually homogeneous, requiring additional HAT agents and can only activate one substrate. Here, we constructed two crystalline porous materials, Zr/Hf-NDI, which possess excellent light absorbing capacity and a confined radical microenvironment, making them able to integrate PS, HAT, and SET processes to simultaneously activate two substrates. Thus, as heterogeneous photocatalysts, they exhibited excellent catalytic performance for the carbon radical-mediated cross-coupling reaction between alcohols and o-phenylenediamine (OPD) to synthesize benzimidazoles (yield > 99%). More importantly, they displayed very good substrate compatibility, especially for OPD substrates with electron-withdrawing groups, even surpassing those of noble metal catalysts. In situ characterizations combined with theoretical calculations showed that the high activity of these catalysts arose from: (i) the metal-oxo clusters and radical NDI˙- ligands can form hydrogen bonding traction activation for the alcohol substrate, and thus facilitate it to generate key intermediate α-carbon radical through a HAT process; (ii) the OPD substrate, acting as an electron donor, forms strong D-A interaction with the NDI ligand and activates the NDI and itself into radicals NDI˙- and OPD˙+, respectively, via an SET process, further promoting the reaction. To the best of our knowledge, this is the best performing crystalline porous catalyst for photocatalytic carbon radical-induced benzimidazole synthesis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Hydrogen Atom Transfer Promoted by Carbon-Centered Biradicals via Energy Transfer Catalysis.Acc Chem Res. 2025 Jul 1;58(13):2028-2045. doi: 10.1021/acs.accounts.5c00228. Epub 2025 Jun 9. Acc Chem Res. 2025. PMID: 40490849
-
Organic Synthesis Away from Equilibrium: Contrathermodynamic Transformations Enabled by Excited-State Electron Transfer.Acc Chem Res. 2024 Jul 2;57(13):1827-1838. doi: 10.1021/acs.accounts.4c00227. Epub 2024 Jun 21. Acc Chem Res. 2024. PMID: 38905487 Free PMC article.
-
Interventions targeted at women to encourage the uptake of cervical screening.Cochrane Database Syst Rev. 2021 Sep 6;9(9):CD002834. doi: 10.1002/14651858.CD002834.pub3. Cochrane Database Syst Rev. 2021. PMID: 34694000 Free PMC article.
-
Does Augmenting Irradiated Autografts With Free Vascularized Fibula Graft in Patients With Bone Loss From a Malignant Tumor Achieve Union, Function, and Complication Rate Comparably to Patients Without Bone Loss and Augmentation When Reconstructing Intercalary Resections in the Lower Extremity?Clin Orthop Relat Res. 2025 Jun 26. doi: 10.1097/CORR.0000000000003599. Online ahead of print. Clin Orthop Relat Res. 2025. PMID: 40569278
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
References
-
- Twilton J. Le C. Zhang P. Shaw M. H. Evans R. W. MacMillan D. W. C. Nat. Rev. Chem. 2017;1:1–19. doi: 10.1038/s41570-017-0052. - DOI
- Yi H. Zhang G. Wang H. Huang Z. Wang J. Singh A. K. Lei A. Chem. Rev. 2017;117:9016–9085. doi: 10.1021/acs.chemrev.6b00620. - DOI - PubMed
- Ji G. Zhao L. Wei J. Cai J. He C. Du Z. Cai W. Duan C. Angew. Chem., Int. Ed. 2021;61:e202114490. doi: 10.1002/anie.202114490. - DOI - PubMed
- Liang R.-R. Han Z. Cai P. Yang Y. Rushlow J. Liu Z. Wang K.-Y. Zhou H.-C. J. Am. Chem. Soc. 2024;146:14174–14181. doi: 10.1021/jacs.4c03038. - DOI - PMC - PubMed
-
- Wang X. He J. Wang Y.-N. Zhao Z. Jiang K. Yang W. Zhang T. Jia S. Zhong K. Niu L. Lan Y. Chem. Rev. 2024;124:10192–10280. doi: 10.1021/acs.chemrev.4c00188. - DOI - PubMed
- Feng K. Raguram E. R. Howard J. R. Peters E. Liu C. Sigman M. S. Buchwald S. L. J. Am. Chem. Soc. 2024;146:26609–26615. doi: 10.1021/jacs.4c09667. - DOI - PMC - PubMed
- Zhao X. Zhu X. Wang K. Lv J. Chen S. Yao G. Lang J. Lv F. Pu Y. Yang R. Zhang B. Jiang Z. Wan Y. Nat. Commun. 2022;13:4180. doi: 10.1038/s41467-022-31967-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

