A double-edged effect of hypoxia on astrocyte-derived exosome releases
- PMID: 40469772
- PMCID: PMC12135208
- DOI: 10.3389/ebm.2025.10559
A double-edged effect of hypoxia on astrocyte-derived exosome releases
Erratum in
-
Corrigendum: A double-edged effect of hypoxia on astrocyte-derived exosome releases.Exp Biol Med (Maywood). 2025 Jul 23;250:10735. doi: 10.3389/ebm.2025.10735. eCollection 2025. Exp Biol Med (Maywood). 2025. PMID: 40771572 Free PMC article.
Abstract
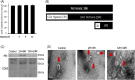
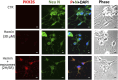
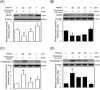
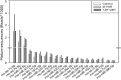
Exosomes are the smallest extracellular vesicles secreted from cells, carrying different cargos, including nucleic acids, proteins and others which transfer from cells to cells. The properties of exosomes depend on the donor cells. Hypoxia, referring to a sublethal and insufficient oxygen supply, reportedly influences exosome secretion of hypoxic cells. In the present study, we focused on the effects of hypoxia on exosomes obtained from CTX-TNA2 astrocyte cells exposed to different durations of hypoxia followed by normoxia as a model of hypoxic preconditioning. To evaluate the functions of exosomes, primary cultured cortical neurons were treated with hemin, a potent neurotoxin. Our sulforhodamine B assay showed that incubation of hemin (30 μM) consistently induced neuronal death. Co-incubation of exosomes from CTX-TNA2 cells subjected to 2 hr-hypoxia plus 6 hr-renormoxia (2H/6R exosomes), but not 12 hr-hypoxia plus 24 hr-renormoxia (12H/24R exosomes), attenuated hemin-induced cell death and reduction in growth associated protein 43 level (a biomarker of neurite outgrowth). Western blot assay demonstrated that 2H/6R exosomes attenuated hemin-induced elevations in inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) levels (two proinflammatory biomarkers) as well as heme oxygenase-1 (HO-1). In contrast, 12H/24R exosomes did not alter hemin-induced elevation in HO-1 but further augmented hemin-induced increases in iNOS and COX-2. Moreover, 2H/6R exosomes attenuated hemin-induced reduction in glutathione hydroperoxidase 4 (a biomarker of ferroptosis) and elevation in active caspase 3 (a biomarker of apoptosis) while 12H/24R exosomes did not effectively alter hemin-induced programed cell death. In conclusion, our study showed that 2H/6R exosomes possessed neuroprotective activities while 12H/24R exosomes had mild pro-inflammatory activities, suggesting that different hypoxic preconditionings influenced CTX-TNA2 cells which then secreted exosomes with differential biological activities. These findings highlight a double-edged role of hypoxia on exosome functions.
Keywords: CTX-TNA2; double-edged role; exosomes; hemin; hypoxic preconditioning.
Copyright © 2025 Tseng, Huang, Lin and Lin.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

