Safety and Effectiveness of Oral Glyburide Suspension in Neonatal Diabetes Mellitus: French Retrospective Cohort Study
- PMID: 40469835
- PMCID: PMC12134890
- DOI: 10.1210/jendso/bvaf083
Safety and Effectiveness of Oral Glyburide Suspension in Neonatal Diabetes Mellitus: French Retrospective Cohort Study
Abstract
Context: Neonatal diabetes mellitus (NDM) is a rare condition usually related to an identifiable genetic cause. Sulfonylurea therapy can ensure metabolic control, obviating the need for insulin while also improving neurodevelopmental outcomes. An oral glyburide suspension (OGS; AMGLIDIA) designed for pediatric use was introduced recently to eliminate the drawbacks of using crushed tablets.
Objective: To evaluate the long-term effectiveness and safety of the OGS for NDM.
Design: Retrospective cohort study.
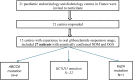
Setting: Fifteen centers in France.
Patients: Consecutive patients started on OGS for NDM in 2015 through 2024.
Intervention: OGS therapy.
Main outcome measures: Glycated hemoglobin (HbA1c) values during OGS therapy; growth; and serious adverse events.
Results: Of 27 patients, 22 had KCNJ11 mutations, 4 had ABCC8 mutations, and 1 had a 6q24 anomaly. Median follow-up during OGS therapy was 2.7 years (range, 2 months-9 years). At baseline, median HbA1c was 6.5% (5.8-7.9) overall and 6.5%, 6.4%, and 8.9% in the KCNJ11, ABCC8, and 6q24 subgroups, respectively. The median starting glyburide dose was 0.15 mg/kg/day (range, 0.1-0.185). HbA1c decreased nonsignificantly over time in all subgroups (P = .382), prompting in a small OGS dosage decrease. The last recorded HbA1c value was 6.3%, 5.9%, and 7.4% in the KCNJ11, ABCC8, and 6q24 subgroups, respectively. Serious adverse events were rare, with hypoglycemia in 2 patients during periods of decreased food intake and diarrhea in 1 patient.
Conclusion: The OGS was effective in maintaining excellent metabolic control in the long term. The safety profile was good. The OGS should be considered for the first-line treatment of NDM.
Keywords: AMGLIDIA; K-ATP channels; long-term efficacy; neonatal diabetes mellitus; pediatric oral glyburide suspension; safety.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Endocrine Society.
Figures



References
-
- Babenko AP, Polak M, Cavé H, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355(5):456‐466. - PubMed
-
- Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350(18):1838‐1849. - PubMed
-
- Busiah K, Drunat S, Vaivre-Douret L, et al. Neuropsychological dysfunction and developmental defects associated with genetic changes in infants with neonatal diabetes mellitus: a prospective cohort study. Lancet Diabetes Endocrinol. 2013;1(3):199‐207. - PubMed
-
- Le Bourgeois F, Beltrand J, Baz B, et al. Long-term metabolic and socioeducational outcomes of transient neonatal diabetes: a longitudinal and cross-sectional study. Diabetes Care. 2020;43(6):1191‐1199. - PubMed
LinkOut - more resources
Full Text Sources
