Res-ECA-UNet++: an automatic segmentation model for ovarian tumor ultrasound images based on residual networks and channel attention mechanism
- PMID: 40470046
- PMCID: PMC12133535
- DOI: 10.3389/fmed.2025.1589356
Res-ECA-UNet++: an automatic segmentation model for ovarian tumor ultrasound images based on residual networks and channel attention mechanism
Abstract
Objective: Ultrasound imaging has emerged as the preferred imaging modality for ovarian tumor screening due to its non-invasive nature and real-time dynamic imaging capabilities. However, in many developing countries, ultrasound diagnosis remains dependent on specialist physicians, where the shortage of skilled professionals and the relatively low accuracy of manual diagnoses significantly constrain screening efficiency. Although deep learning has achieved remarkable progress in medical image segmentation in recent years, existing methods still face challenges in ovarian tumor ultrasound segmentation, including insufficient robustness, imprecise boundary delineation, and dependence on high-performance hardware facilities. This study proposes a deep learning-based automatic segmentation model, Res-ECA-UNet++, designed to enhance segmentation accuracy while alleviating the strain on limited healthcare resources.
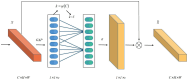
Methods: The Res-ECA-UNet++ model employs UNet++ as its fundamental architecture with ResNet34 serving as the backbone network. To effectively address the vanishing gradient problem in deep networks, residual modules are incorporated into the skip connections between the encoding and decoding processes. This integration enhances feature extraction efficiency while improving model stability and generalization capabilities. Furthermore, the ECA-Net channel attention mechanism is introduced during the downsampling phase. This mechanism adaptively emphasizes tumor region-related channel information through global feature recalibration, thereby improving recognition accuracy and localization precision for tumor areas.
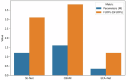
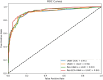
Results: Based on clinical ultrasound datasets of ovarian tumors, experimental results demonstrate that Res-ECA-UNet++ achieves outstanding performance in clinical validation, with a Dice coefficient of 95.63%, mean Intersection over Union (mIoU) of 91.84%, and accuracy of 99.75%. Compared to the baseline UNet, Res-ECA-UNet++ improves these three metrics by 0.45, 4.42, and 1.57%, respectively. Comparative analyses of ROC curves and AUC values further indicate that Res-ECA-UNet++ exhibits superior segmentation accuracy and enhanced generalization capabilities on the test set. In terms of computational efficiency, the inference time of Res-ECA-UNet++ meets clinical real-time requirements on both high-end and low-end hardware, demonstrating its suitability for deployment on resource-constrained devices. Additionally, comparative experiments on the public OTU2D dataset validate the model's superior segmentation performance, highlighting its strong potential for practical applications.
Conclusion: The proposed Res-ECA-UNet++ model demonstrates exceptional accuracy and robustness in the segmentation of ovarian tumor ultrasound images, highlighting its potential for clinical application. Its ability to enhance segmentation precision and aid clinicians in diagnosis underscores broad prospects for practical implementation. Future research will focus on optimizing the model architecture to further improve its adaptability to diverse pathological types and imaging characteristics, thereby expanding its clinical diagnostic utility.
Keywords: UNet++; attention mechanism; medical image segmentation; ovarian tumor; residual networks.
Copyright © 2025 Wei, Hu and Tan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alcázar JL, Pérez-Vidal JR, Tameish S, Chacón E, Manzour N, Mínguez JÁ, et al. Ultrasound for assessing tumor spread in ovarian cancer. A systematic review of the literature and meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2024) 292:194–200. doi: 10.1016/j.ejogrb.2023.11.017, PMID: - DOI - PubMed
-
- Cai G, Huang F, Gao Y, Li X, Chi J, Xie J, et al. Artificial intelligence-based models enabling accurate diagnosis of ovarian cancer using laboratory tests in China: a multicentre, retrospective cohort study. Lancet Digital Health. (2024) 6:e176–86. doi: 10.1016/S2589-7500(23)00245-5, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

