Request of endocrinology and metabolism journals for data sharing statements in clinical trial reports: a survey
- PMID: 40470047
- PMCID: PMC12133921
- DOI: 10.3389/fmed.2025.1518399
Request of endocrinology and metabolism journals for data sharing statements in clinical trial reports: a survey
Abstract
Background: To enhance reproducibility and transparency, the International Committee of Medical Journal Editors (ICMJE) required that all trial reports submitted after July 2018 must include a data sharing statement (DSS). Accordingly, emerging biomedical journals required trial authors to include a DSS in submissions for publication if trial reports were accepted. Nevertheless, it was unclear whether endocrinology and metabolism journals had this request for DSS of clinical trial reports. Therefore, we aimed to explore whether endocrinology and metabolism journals requested DSS in clinical trial submissions, and their compliance with the declared request in published trial reports.
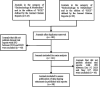
Methods: Journals that were from the category of "Endocrinology & Metabolism" defined by Journal Citation Reports (JCR, as of June 2023) and published clinical trial reports between 2019 and 2022, were included for analysis. The primary outcome was whether a journal explicitly requested a DSS in its manuscript submission instructions for clinical trials, which was extracted and verified in December 2023. We also evaluated whether these journals indeed included a DSS in their published trial reports that were published between December 2023 and May 2024.
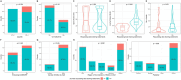
Results: A total of 141 endocrinology and metabolism journals were included for analysis, among which 125 (88.7%) requested DSS in clinical trial submissions. Journals requesting DSS had a significantly lower JCR quartile and higher impact factor when compared with those journals without DSS request. Among the 90 journals requesting DSS, 14 (15.6%) journals indeed did not publish any DSS in their published trial reports between December 2023 and May 2024.
Conclusion: Over 10% of endocrinology and metabolism journals did not request DSS in clinical trial submissions. More than 15% of the journals declaring to request DSS from their submission instructions, did not publish DSS in their published trial reports. More efforts are needed to improve the practice of endocrinology and metabolism journals in requesting and publishing DSS of clinical trial reports.
Keywords: ICMJE; clinical trial; data sharing; endocrinology; metabolism.
Copyright © 2025 Liu, Chen, Zhang, Bai, Kang, Li and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

