Advancing Cancer Treatment: Innovative Materials in PDT and Diagnostic Integration
- PMID: 40470109
- PMCID: PMC12136068
- DOI: 10.2147/IJN.S514937
Advancing Cancer Treatment: Innovative Materials in PDT and Diagnostic Integration
Abstract
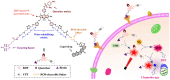
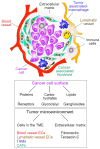
The diagnosis and treatment of cancers have become a significant challenge in overcoming malignant diseases. Early detection of tumors and timely targeted therapy can greatly impede the rapid deterioration of cancers. In recent years, nano-systems based on photodynamic materials have shown great progress in tumor diagnosis and treatment applications. With the continuous exploration of tumor-specific targets and the development of photodynamic nanoparticles, the generation of new nanoparticles that are target-specific, highly sensitive, and biosafe for integrated diagnosis and therapy is realistic. This review introduces the rational basis for photosensitizer-based materials for integrating cancer diagnosis and anti-cancer therapy, types and characteristics of organic and inorganic photosensitizers currently used for PDT treatment, photosensitive nano-materials with dual detection and therapeutic properties the advancement in developing photo-dynamic nano-systems showing potential in integrated diagnosis and therapeutic applications. We also introduce current strategies for optimizing nano-systems with the properties for enhancing targeting ROS release and accurate imaging, combining therapeutic efficacy, as well as biosafety of the integrative materials for PDT application, providing references for the coordinated optimization of photosensitizer design and clinical translation.
Keywords: advanced imaging technology; integrated diagnosis and treatment; novel nano-systems; photodynamic therapy; precision anti-cancer therapy; tumor targeting.
Plain language summary
The integration of PDT with advanced diagnostic modalities represents a transformative approach in cancer theranostics, as evidenced by the comprehensive exploration of photosensitizer-based nanomaterials in this review. By leveraging the unique photophysical properties of organic and inorganic photosensitizers, researchers have developed innovative nano-systems capable of simultaneous tumor detection and targeted therapy. Key advancements include the rational design of aggregation-induced emission photosensitizers (AIE-PS) with redshifted absorption spectra, hypoxia-responsive nanomaterials for enhanced ROS generation, and multifunctional composites such as UCNP@MOF hybrids that address tumor microenvironment limitations. These systems demonstrate improved targeting precision, reduced off-target effects, and synergistic therapeutic outcomes when combined with chemotherapy or immunotherapy. Despite these strides, challenges persist in optimizing light penetration depth, mitigating photobleaching, and ensuring biosafety during clinical translation. The development of oxygen-independent Type III photosensitizers and stimuli-responsive delivery systems offers promising avenues to overcome hypoxia-related barriers. Furthermore, the integration of MOFs and UCNPs highlights the potential for real-time imaging-guided therapy. Future efforts should prioritize scalable synthesis, rigorous toxicological profiling, and combinatorial strategies to enhance therapeutic efficacy while minimizing systemic toxicity. By bridging nanotechnology, materials science, and clinical oncology, next-generation photodynamic platforms hold immense potential to redefine precision medicine in oncology and beyond. While PDT nanomaterials offer revolutionary potential in cancer theranostics, addressing toxicity, stability, and clinical scalability is critical. Integrating PDT with complementary modalities (chemotherapy, immunotherapy) and advancing TME-responsive designs will bridge the gap between preclinical innovation and clinical application.
© 2025 Wang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

