Dual Bronchodilators in Bronchiectasis Study: a randomised controlled trial
- PMID: 40470153
- PMCID: PMC12134924
- DOI: 10.1183/23120541.01079-2024
Dual Bronchodilators in Bronchiectasis Study: a randomised controlled trial
Abstract
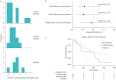
This study comparing exacerbations rates for dual bronchodilator therapy, triple therapy and placebo in bronchiectasis cannot provide definitive evidence. However, results suggest signs of of efficacy: a larger trial may provide valuable clinical evidence. https://bit.ly/4fuuHHX.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: N. Wilson, M. Morton and A.B. Konkoth report receiving grants from the National Institute of Health and Care Research (NIHR). J. Wason reports being funded by a NIHR Research Professorship (NIHR301614), being a panel member on NIHR Healthcare Technology Assessment Funding Committee (2020–2024) selection panel Chair for NIHR Undergraduate Internship scheme (2024) and receiving GSK funding for a PhD studentship. L. Ternent reports receiving grants from the NIHR. J. Steer reports received grants from Chiesi, personal payments and support for attending meetings/travel from Astra Zeneca, and personal payments from UK Cardiopulmonary Taskforce. G. Devereux reports receiving grants from the NIHR. J.D. Chalmers reports receiving grants from NIHR, AstraZeneca, Boehringer Ingelheim, GSK, Zambon, Insmed and Gilead; receiving personal fees from AstraZeneca, Boehringer Ingelheim, GSK, Zambon, Insmed, Novartis and Cheisi; and is an associate editor of this journal. A.T. Hill reports being Chair of the British Thoracic Society Standards of Care Committee. C.S. Haworth reports receiving grants from NIHR; receiving consulting fees from 30 Technology, Astra Zeneca, CSL Behring, Chiesi, Infex, Insmed, Janssen, LifeArc, Meiji, Mylan, Pneumagen, Shionogi, Vertex and Zambon; receiving fees for advisory and educational work from Chiesi, Insmed, Mylan and Zambon; and holding stock/stock option in Pneumagen. J.R. Hurst reports receiving grants from AstraZeneca, and receiving fees for advisory and educational work from AstraZeneca, Boehringer Ingleheim, Chiesi, GSK, Novartis and Sanofi. A. De Soyza reports receiving grant awards from NIHR, and grant support from Astra Zeneca, Bayer, Gilead, Chiesi, Pfizer and GSK; and receiving speaker fees for bureau/advisory committee work from Astra Zeneca, Bayer, Gilead, Chiesi, Pfizer, GSK, 30 T Pharmaceuticals and Insmed. All other authors have nothing to disclose.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
