Chronic lung allograft dysfunction after lung transplantation: prevention, diagnosis and treatment in 44 European centres
- PMID: 40470157
- PMCID: PMC12134928
- DOI: 10.1183/23120541.00675-2024
Chronic lung allograft dysfunction after lung transplantation: prevention, diagnosis and treatment in 44 European centres
Abstract
Background: There are limited data on optimal management of chronic lung allograft dysfunction (CLAD). We aimed to describe the variability of diagnostic and therapeutic practices in Europe.
Methods: A structured questionnaire was sent to 71 centres in 24 countries. Questions were related to contemporary clinical practices for workup, monitoring and treatment of CLAD. The number of lung transplant procedures and patients in follow-up were collected.
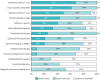
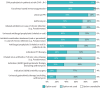
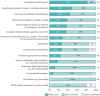
Results: 44 centres (62%) responded from 20 countries, representing 74% of European activity. The prevalence of CLAD was estimated at 9.1 cases per million population (25th and 75th percentiles of 4.4, 15.7). Preferred initial workup for probable CLAD consisted of chest computed tomography (CT) (inspiratory 91% and expiratory 74%), donor-specific antibody (DSA) measurement (86%), bronchoalveolar lavage (BAL) (85%) and transbronchial biopsy (81%). For monitoring of definite CLAD, inspiratory CT (67%), DSA (61%) and BAL (43%) were preferred. Body plethysmography was unavailable for 16% of cases. Prophylaxis was based on preventing infections (cytomegalovirus 99%, inhaled antibiotics 70% and antifungals 65%), tacrolimus-based immunosuppression (96%), azithromycin (72%) and universal proton pump inhibitor treatment (84%). First-line treatment of CLAD was based on azithromycin (82%) and steroid augmentation (74%). Photopheresis was used in 26% of cases.
Conclusion: Current European practice CLAD detection is based on spirometry, inspiratory CT and DSA, with limited access to plethysmography and expiratory CT. Prophylactic treatment is based on azithromycin, tacrolimus-based immunosuppression and treatment of risk factors. No single treatment strategy is universally used, highlighting the need for an effective treatment of CLAD. The preferred first-line strategy is azithromycin and steroid augmentation.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: J. Gottlieb reports grants from Deutsche Forschungsgemeinschaft, Zambon and the German Center of Lung Research (DZL); personal fees from Novartis, AstraZeneca, CSL Behring, Sanofi and Moderna, all outside the submitted work. He served as a member of the SCAN CLAD Study data safety monitoring board as a nonfinancial disclosure. Conflict of interest: R. Vos reports grants from Research Foundation-Flanders, Cystic Fibrosis Foundation and AstraZeneca; nonfinancial support from Incyte, Zambon and Sanofi; and personal fees from AstraZeneca, GlaxoSmithKline, Zambon, Takeda, Shionogi and Sanofi, all outside the submitted work. Conflict of interest: M. Hellemons is an associate editor of this journal. Conflict of interest: J. Magnusson reports personal fees from Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, Takada Pharma, Vicore Pharma and Mallinckrodt, outside the submitted work. Conflict of interest: V. Ennekes reports personal fees from Astra Zeneca outside the submitted work. Conflict of interest: P. Riddell is an associate editor of this journal. Conflict of interest: M. Perch reports personal fees from Zambon, Takeda, AstraZeneca, TFF and PulmonX; other support from Therakos; and support from Ryme medical, all outside the submitted work; and is a member of the steering committee of the eCLAD study. Conflict of interest: F.M. Carlier reports travel grants from Takeda outside the present work. Conflict of interest: A. Fisher reports grants and personal fees from Therakos, other support from Sanofi, and grants from Chiesi, all outside the submitted work. Conflict of interest: B. Saez-Gimenez reports institutional research grants from Janssen Pharmaceuticals; fees for consultancy from Janssen Pharmaceuticals and Chiesi Spain; speaker fees from Janssen Pharmaceuticals and CareDx; and travel grants from Chiesi Spain. All disclosures are unrelated to the current work. Conflict of interest: The other authors have nothing to disclose.
Figures

References
-
- Chambers DC, Perch M, Zuckermann A, et al. . The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report - 2021; Focus on recipient characteristics. J Heart Lung Transplant 2021; 40: 1060–1072. doi:10.1016/j.healun.2021.07.021 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
