This is a preprint.
Extracellular Vesicle Profiling Reveals Novel Autism Signatures in Patient-Derived Forebrain Organoids
- PMID: 40470213
- PMCID: PMC12136235
- DOI: 10.21203/rs.3.rs-6573757/v1
Extracellular Vesicle Profiling Reveals Novel Autism Signatures in Patient-Derived Forebrain Organoids
Abstract
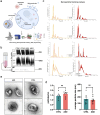
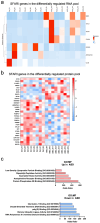
Autism Spectrum Disorder (ASD) affects 1 percent of the world's population with an increased prevalence of 178 percent since 2000. Although altered synaptic function putatively accounts for many of the abnormalities seen in ASD, the specific molecular mechanisms underlying this disorder remain poorly defined. A growing body of evidence suggests that extracellular vesicles (EVs), specifically exosomes, play a critical role in cellular communication within the brain. While they have been implicated in various types of diseases from cancer to neurodegeneration, their involvement in ASD remains largely unexplored. In this study, we utilized patient-derived cortical organoid models to characterize EVs secreted by human three-dimensional (3D) tissue and defined their cargo. Our study reports, for the first time, alterations in ASD organoid-derived EVs in comparison to healthy control cortical EVs. By utilizing small RNA sequencing, proteomics, nanoparticle tracking and microscopy, we provide a comprehensive characterization of the cargo carried by EVs secreted from human 3D forebrain models. Our findings reveal substantial differences both in the RNA and protein content of ASD-derived EVs, providing insight into disease mechanisms as well as highlighting the potential of exosome-based diagnostics and therapies for ASD.
Conflict of interest statement
Conflict of interest statement The authors report no conflict of interest or commercial interests related to the manuscript. Additional Declarations: The authors have declared there is NO conflict of interest to disclose
Figures




References
-
- Ramaswami G., & Geschwind D. H. (2018). Genetics of autism spectrum disorder. In Handbook of clinical neurology (Vol. 147, pp. 321–329). - PubMed
-
- Gentilini D., Cavagnola R., Possenti I., Calzari L., Ranucci F., Nola M., Olivola M., Brondino N., & Politi P. (2023). Epigenetics of autism spectrum disorders: A multi-level analysis combining epi-signature, age acceleration, epigenetic drift, and rare epivariations using public datasets. Current Neuropharmacology, 21(11), 2362–2373. 10.2174/1570159X21666230725142338 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

