Novel cancer stem cell-targeted retinoid ZSH-512 impedes colorectal cancer progress via KDM3A-mediated epigenetic reprogramming
- PMID: 40470327
- PMCID: PMC12130993
- DOI: 10.1016/j.xinn.2025.100831
Novel cancer stem cell-targeted retinoid ZSH-512 impedes colorectal cancer progress via KDM3A-mediated epigenetic reprogramming
Abstract
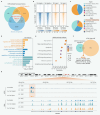
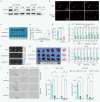
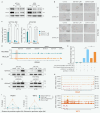
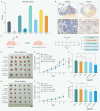
Advanced colorectal cancer (CRC) exhibits weak responses to multiple therapies, primarily due to the presence of cancer stem cells (CSCs), which drive high recurrence rates, metastasis, and drug resistance. We have previously systematically conducted CSC-targeted compound discovery and evaluation studies to inhibit CSC-mediated tumorigenesis and metastasis. Here, we identified ZSH-512, a novel synthetic retinoid that selectively targets retinoic acid receptor (RAR)γ, demonstrating its ability to effectively inhibit CRC-CSCs and patient-derived organoids (PDOs) in vitro and significantly reduce CSC-mediated tumor formation and liver metastasis in mouse models without noticeable toxicity. Mechanistically, integrated analysis of Assay for Transposase-Accessible Chromatin sequencing (ATAC-seq) and RNA sequencing (RNA-seq) revealed that ZSH-512 exerted its effect by modulating the RARγ-KDM3A axis to mediate epigenetic reprogramming and broadly suppress stemness-related signaling pathways, including Wnt, Hippo, and Hedgehog. ZSH-512 efficiently inhibited tumorigenesis in CRC-patient-derived tumor xenografts (PDXs) with high KDM3A expression, suggesting KDM3A as a potential predictive biomarker. Collectively, ZSH-512 is a promising therapeutic candidate for targeting CRC-CSCs with high efficacy.
Keywords: KDM3A; ZSH-512; cancer stem cell; colorectal cancer; epigenetic reprogramming; retinoid; tumor-repopulating cell.
© 2025 The Authors.
Conflict of interest statement
The number of the patent registration related to this work is ZL202211618530.3.
Figures





Similar articles
-
ZSH-2208: A novel retinoid with potent anti-tumour effects on ESCC stem cells via RARγ-TNFAIP3 axis.Clin Transl Med. 2025 Jan;15(1):e70148. doi: 10.1002/ctm2.70148. Clin Transl Med. 2025. PMID: 39724264 Free PMC article.
-
MACC1 Regulates LGR5 to Promote Cancer Stem Cell Properties in Colorectal Cancer.Cancers (Basel). 2024 Jan 31;16(3):604. doi: 10.3390/cancers16030604. Cancers (Basel). 2024. PMID: 38339354 Free PMC article.
-
Jiedu Xiaozheng Yin extract targets cancer stem cells by Wnt signaling pathway in colorectal cancer.J Ethnopharmacol. 2025 Jan 30;337(Pt 1):118710. doi: 10.1016/j.jep.2024.118710. Epub 2024 Aug 26. J Ethnopharmacol. 2025. PMID: 39197803
-
Emerging Prospects for the Study of Colorectal Cancer Stem Cells using Patient-derived Organoids.Curr Cancer Drug Targets. 2022;22(3):195-208. doi: 10.2174/1568009622666220117124546. Curr Cancer Drug Targets. 2022. PMID: 35078398 Review.
-
Crosstalk between colorectal CSCs and immune cells in tumorigenesis, and strategies for targeting colorectal CSCs.Exp Hematol Oncol. 2024 Jan 22;13(1):6. doi: 10.1186/s40164-024-00474-x. Exp Hematol Oncol. 2024. PMID: 38254219 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

