CONFIDENCE treatment success: long-term real-world effectiveness and safety of ocrelizumab in Germany
- PMID: 40470489
- PMCID: PMC12135804
- DOI: 10.3389/fneur.2025.1564327
CONFIDENCE treatment success: long-term real-world effectiveness and safety of ocrelizumab in Germany
Abstract
Background: Early high-efficacy treatment for people with relapsing multiple sclerosis (pwRMS) may provide better long-term outcomes compared with the escalation strategy. In this study, we present an analysis of treatment success and safety from the CONFIDENCE study in a real-world cohort of pwRMS treated with ocrelizumab in different treatment lines for up to 5.5 years.
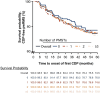
Methods: The ongoing German non-interventional post-authorization safety study, CONFIDENCE (ML39632, EUPAS22951), evaluates the long-term safety and effectiveness of therapy in pwMS newly treated with ocrelizumab or other disease-modifying therapies for up to 10 years. This analysis presents CONFIDENCE treatment success (proportion of people with no clinical disease activity measured by relapse or disease progression and no treatment discontinuation due to adverse event [AE] or lack of therapeutic effectiveness), confirmed disability progression (CDP), annualized relapse rates, and safety in pwRMS stratified by the number of previous MS therapies (PMSTs).
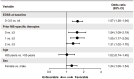
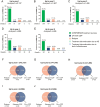
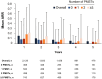
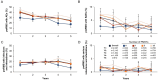
Results: At the data cutoff (11 October 2023), the full analysis set included 2,261 pwRMS treated with ≥1 dose of ocrelizumab. At baseline, the mean age (SD) of the participants was 41.16 (11.39) years (treatment-naïve, 39.19 [12.95] years; ≥3 PMSTs, 42.80 [10.08] years), and the mean Expanded Disability Status Scale (EDSS) score was 3.08 (1.86) (treatment-naïve, 2.37 [1.54]; ≥3 PMSTs, 3.57 [1.90]). Overall, 58.4% of pwRMS with continuous treatment achieved CONFIDENCE treatment success from baseline until year 5 (74.0 and 50.3% of pwRMS with 0 and ≥3 PMSTs). The main reasons for not achieving CONFIDENCE treatment success were relapse and CDP, while treatment discontinuation due to AEs or lack of effectiveness played a minor role. The proportion of pwRMS with AEs did not increase with longer treatment duration and tended to be higher with more PMSTs. The spectrum of AEs was similar across treatment lines, and no new or unexpected AEs were observed.
Conclusion: CONFIDENCE treatment success remained high over 5 years of ocrelizumab treatment, even among people with RMS (pwRMS) with a higher number of PMSTs. Only a small proportion of pwRMS discontinued treatment due to AEs. These results support early intervention with high-efficacy ocrelizumab treatment to optimize long-term outcomes for pwRMS.
Trial registration: https://catalogues.ema.europa.eu/node/3142/administrative-details, identifiers ML39632 and EUPAS22951.
Keywords: Relapsing multiple sclerosis; effectiveness; humanized monoclonal anti-CD20 antibody; neurodegenerative diseases; non-interventional study (NIS); ocrelizumab; real-world cohort; treatment success.
Conflict of interest statement
MB received honoraria for lecturing, consulting and/or travel expenses for attending meetings from Biogen, Bristol Myers Squibb, Das Fortbildungskolleg, Merck, Novartis, RG Ärztefortbildung, Roche, Sandoz, Sanofi, Teva and Viatris. MW receives research support from the Deutsche Forschungsgemeinschaft (DFG; WE 3547/5-1, WE3547/7-1; in association with SFB TRR 274), from Novartis, TEVA, Biogen Idec, Roche, Merck and the ProFutura Programm of the Universitätsmedizin Göttingen. MW is serving as an editor for PLoS One. He received travel funding and/or speaker honoraria from Biogen Idec, Merck Serono, Novartis, Roche, TEVA, Bayer and Genzyme. SM receives honoraria for lecturing, travel expenses and for attending meetings from Academy 2, Argenx, Alexion, Almirall, Amicus Therapeutics Germany, AstraZeneca, Bayer Health Care, Biogen, BioNtech, BMS, Celgene, Datamed, Demecan, Desitin, Diamed, Diaplan, DIU Dresden, DPmed, Gen Medicine and Healthcare products, Genzyme, Hexal AG, IGES, Impulze GmbH, Janssen Cilag, KW Medipoint, MedDay Pharmaceuticals, Medmile, Merck Serono, MICE, Mylan, Neuraxpharm, Neuropoint, Novartis, Novo Nordisk, ONO Pharma, Oxford PharmaGenesis, QuintilesIMS, Roche, Sanofi, Springer Medizin Verlag, STADA, Chugai Pharma, Teva, UCB, Viatris, Wings for Life international and Xcenda. His research is funded by the German Ministry for Education and Research (BMBF), German Federal Institute for Risk Assessment (BfR), German Research Foundation (DFG), Else Kröner Fresenius Foundation, Gemeinsamer Bundesausschuss (G-BA), German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology, Ministry of Culture and Science of the State of North Rhine-Westphalia, The Daimler and Benz Foundation, Multiple Sclerosis Society North Rhine-Westphalia Regional Association (dmsg), Peek & Cloppenburg Düsseldorf Foundation, Hempel Foundation for Science, Art and Welfare, German Alzheimer Society e.V. Dementia self-help and Alexion, Almirall, Amicus Therapeutics Germany, Argenx, Bayer Vital GmbH, BGP Products Operations (Viatris Company), Biogen, BMS, Demecan, Diamed, DGM e.v., Fresenius Medical Care, Genzyme, Gesellschaft von Freunden und Förderern der Heinrich-Heine-Universität Düsseldorf e.V., HERZ Burgdorf, Hexal, Janssen, Merck Serono, Novartis, Novo Nordisk Pharma, ONO Pharma, Roche and Teva. SB is an employee of Roche Pharma AG and shareholder of F. Hoffmann La Roche AG. SH-S is an employee of Roche Pharma AG. PD is an employee of F. Hoffmann La Roche AG and shareholder of F. Hoffmann La Roche AG. JE is an employee of Roche Pharma AG and shareholder of F. Hoffmann La Roche AG. TZ reports personal fees from Biogen, Roche, Merck, TEVA, NovoNordisk, Neuraxpharm, BMS, Novartis, Sanofi, Sandoz, Viatris; research support from Genzyme, Novartis, Roche, Sanofi, Teva, Novartis, Neuraxpharm. The authors declare that this study received funding from Roche Pharma AG (Grenzach-Wyhlen, Germany). Financial support for medical writing services from Ashfield MedComms GmbH (Mannheim, Germany), an Inizio Company, was provided by Roche Pharma AG (Grenzach-Wyhlen, Germany). The funder had the following involvement in the study: study management, study design, data collection, data analysis and interpretation, writing of this article, and decision to submit it for publication.
Figures


References
-
- Hauser S, Kappos L, Filippi M, Wolinsky J, Weber M, Nicholas J, et al. 10 years of Ocrelizumab treatment in multiple sclerosis: long-term efficacy and safety clinical trial data (S31.005). Neurology. (2024) 102:5124. doi: 10.1212/WNL.0000000000205584 - DOI
LinkOut - more resources
Full Text Sources

