Long-term outcomes in leucine-rich glioma inactivated-1 autoimmune encephalitis and associated biomarkers of inflammation and neuronal and glial injury
- PMID: 40470500
- PMCID: PMC12133544
- DOI: 10.3389/fneur.2025.1583892
Long-term outcomes in leucine-rich glioma inactivated-1 autoimmune encephalitis and associated biomarkers of inflammation and neuronal and glial injury
Abstract
Introduction: Leucine-rich glioma inactivated 1 (LGI1) autoimmune encephalitis (AE) is characterized by seizures, as well as cognitive, memory, and behavioral disturbances. Blood-based biomarkers for inflammation and neuronal and glial injury have been evaluated as potential markers of disease severity and prognosis in AE.
Methods: Patients diagnosed with LGI1 AE, confirmed by cell-based assay, were enrolled and followed prospectively to gather plasma samples for biomarker testing. Biomarkers of neuronal and glial injury included plasma neurofilament light chain (NfL), glial fibrillary acidic protein (GFAP), ubiquitin c-terminal hydrolase L1 (UCHL-1), tau, and cytokine markers of inflammation. Biomarker data were logarithmically transformed and analyzed using longitudinal regression with the ratio of means between LGI1 AE and non-inflammatory controls. Clinical data were collected and correlated with blood-based biomarkers to assess their relationship to disease severity and long-term outcomes.
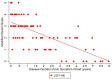
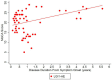
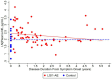
Results: Twenty-one LGI1 AE patients were enrolled from October 2018 to April 2024, and 16 migraine headache patients (56.3% male; average age: 58 years) served as non-inflammatory controls. One LGI1 AE patient in our cohort had a clinical relapse. Modified Rankin Score (mRS) and Montreal Cognitive Assessment (MoCA) improved over time. The mRS at symptom onset was 3.34 and dropped to 0.56 in a 5-year follow-up. Mean MoCA scores were 18.45 at the onset and increased to 29.40 in the 6-year follow-up. The model estimated geometric mean plasma NfL values at disease diagnosis to be 11.86 pg/mL; it was estimated to be 6.07 pg/mL when compared to non-inflammatory controls. The model also estimated the plasma GFAP values to be 77.70 pg/mL; it was estimated to be 36.26 pg/mL when compared to non-inflammatory controls. The trend of clinical improvement is paralleled with a slow decline in NfL and GFAP levels, returning to levels like our control population after 6 and 3 years, respectively. MoCA scores tended to recover more quickly in patients presenting with lower Nfl scores at symptom onset.
Conclusion: Improved clinical symptoms were correlated with improvements in initially high NfL and GFAP levels. In one patient with a clinical relapse, NfL and GFAP levels increased. NfL and GFAP may be useful biomarkers of disease progression in patients with LGI1 AE. However, additional studies are needed to better understand the effects of immunotherapy.
Keywords: autoimmune encephalitis; biomarkers; glial fibrillary acidic protein; leucine-rich glioma inactivated 1; neurofilament light chain.
Copyright © 2025 Borko, Winters, Money, Sillau, Eggmann, Selva, Ritchie, Kammeyer, Owens, Bennett and Piquet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
-
- Lee ST, Abboud H, Irani SR, Nakajima H, Piquet AL, Pittock SJ, et al. . Innovation and optimization in autoimmune encephalitis trials: the design and rationale for the phase 3, randomized study of satralizumab in patients with NMDAR-IgG-antibody-positive or LGI1-IgG-antibody-positive autoimmune encephalitis (CIELO). Front Neurol. (2024) 15:1437913. doi: 10.3389/fneur.2024.1437913, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

