Survival in Duchenne muscular dystrophy in Australia: a 50 year retrospective cohort study
- PMID: 40470523
- PMCID: PMC12136836
- DOI: 10.1016/j.lanwpc.2025.101568
Survival in Duchenne muscular dystrophy in Australia: a 50 year retrospective cohort study
Abstract
Background: There is limited evidence describing the changing natural history of DMD in Australia.
Methods: This retrospective cohort study collated information on clinical management and disease milestones from medical records of males with DMD attending a paediatric hospital between 1973 and 2019 and linked this to information from two adult tertiary hospitals. Data were stratified by decade of birth and Kaplan Meier analyses were conducted to describe median time to key disease milestones.
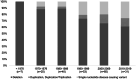
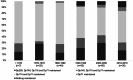
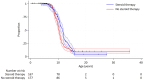
Findings: The cohort included 356 individuals with DMD with year of birth ranging from 1958 to 2014 and median (interquartile range, IQR) follow up time from diagnosis of 10.5 (4.1, 15.7) years. Use of corticosteroids, angiotensin-converting enzyme inhibitors (ACE-I), echocardiography and respiratory support increased over time. Mean age of diagnosis decreased from 6.4 years in those born before 1970 to 3.4 years in those born 2010-2019. Median (IQR) survival increased over time from 18.2 (15.2, 20.4) years in those born before 1970 to 24.0 (20.3, 27.5) years in those born between 1990 and 1999. Increased life expectancy was observed in individuals using corticosteroids, ACE-I and respiratory support.
Interpretation: Survival in individuals with DMD has increased over the last five decades, likely due to changes in clinical management. Given the increased population surviving to adulthood, there is a need to enhance clinical services and surveillance to support neuromuscular disease in Australia, especially in transitional care and adult populations.
Funding: Independent Research Grant, Pfizer Australia.
Keywords: Cohort study; Duchenne muscular dystrophy; Natural history; Survival.
© 2025 The Authors.
Conflict of interest statement
IRW is a member of advisory boards for Avidity, Biogen, Novartis, Percheron Therapeutics, Pfizer, Roche and Solid Biosciences and has received research grant funding from NIH, Fulcrum Therapeutics, FSHD Global and FSHD Society. None are specifically relevant to this project. EMY is PI of the Australian Neuromuscular Diseases Registry and has received research support from TREAT-NMD, MD-NSW, The Daniel Ferguson Foundation, Save our Sons Duchenne Foundation, the Western Australian government, Biogen, Roche, Novartis, PTC therapeutics, and Pfizer, all unrelated to the content of this manuscript. EMY has received honoraria for advisory board participation from Biogen and Roche, including honoraria paid to their institution from Biogen. EMY has also received speaker fees paid to their institution from Biogen and PTC Therapeutics. DP has received honoraria for educational presentations from Novartis and AstraZeneca. KC has received grants from Pfizer unrelated to the content of this manuscript. The remaining authors have no disclosures to declare.
Figures



References
-
- Emery A.E. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul Disord. 1991;1(1):19–29. - PubMed
-
- Mendell J.R., Shilling C., Leslie N.D., et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71(3):304–313. - PubMed
-
- Bushby K., Finkel R., Birnkrant D.J., et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9(1):77–93. - PubMed
-
- Boland B.J., Silbert P.L., Groover R.V., Wollan P.C., Silverstein M.D. Skeletal, cardiac, and smooth muscle failure in Duchenne muscular dystrophy. Pediatr Neurol. 1996;14(1):7–12. - PubMed
-
- Brooke M.H., Fenichel G.M., Griggs R.C., et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39(4):475–481. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

