Multi-system dysregulation in placental malaria contributes to adverse perinatal outcomes in mice
- PMID: 40470930
- PMCID: PMC12234438
- DOI: 10.1128/iai.00021-25
Multi-system dysregulation in placental malaria contributes to adverse perinatal outcomes in mice
Abstract
Sequestration of Plasmodium parasites in the placental vasculature contributes to increased morbidity and mortality in pregnant compared to non-pregnant patients in malaria-endemic regions. In this study, outbred pregnant CD1 mice with semi-allogeneic fetuses were infected with transgenic Plasmodium berghei or mock inoculated by mosquito bite at either embryonic day (E)6 (first trimester-equivalent) or 10 (second trimester-equivalent) and were compared to non-pregnant females. P. berghei-infected mosquitoes had greater biting avidity for E10 dams than uninfected mosquitoes, which was not apparent for E6 dams nor non-pregnant females. Infected E10 dams had greater numbers of parasites than E6 dams in the uterus and spleen, but not in the blood or liver. While parasites were found in placentas, no parasites were present in fetuses. Maternal infection at E6 caused greater maternal morbidity, with greater rates of fetal reabsorption and stillbirths than at E10. Infection at E10 caused adverse offspring outcomes, including growth restriction. To identify possible mechanisms of adverse offspring outcomes, E10 dams were euthanized during peak parasitemia (8 days postinfection [dpi]), and outcomes were compared to mock-infected dams. P. berghei caused significant systemic maternal immune activation with elevated circulating lymphocytes, eosinophils, and neutrophils and splenic cytokine concentrations. P. berghei infection at E10 increased corticosterone and decreased progesterone concentrations, which could contribute to adverse perinatal outcomes through immunomodulation. There were limited changes in the maternal fecal microbiome after P. berghei infection. Mosquito bite infection of outbred dams with P. berghei causes placental malaria and provides a novel, tractable model to investigate therapeutic treatments.
Keywords: Plasmodium; corticosterone; cytokines; microbiome; pregnancy; progesterone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

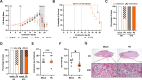
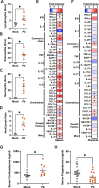
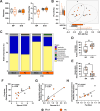
Update of
-
Multi-System Dysregulation in Placental Malaria Contributes to Adverse Perinatal Outcomes in Mice.bioRxiv [Preprint]. 2025 Jan 15:2025.01.15.633265. doi: 10.1101/2025.01.15.633265. bioRxiv. 2025. Update in: Infect Immun. 2025 Jul 8;93(7):e0002125. doi: 10.1128/iai.00021-25. PMID: 39868257 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

