Growth hormone combined with estrogen improves intrauterine adhesion fibrosis by downregulating endometrial microbial citraconic acid to target β-catenin protein
- PMID: 40470939
- PMCID: PMC12282089
- DOI: 10.1128/msystems.01692-24
Growth hormone combined with estrogen improves intrauterine adhesion fibrosis by downregulating endometrial microbial citraconic acid to target β-catenin protein
Abstract
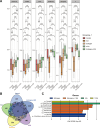
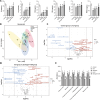
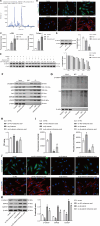
Intrauterine adhesions (IUAs) are a major cause of secondary infertility. This study aimed to investigate the therapeutic effects and mechanisms of combined recombinant rat growth hormone (rrGH) and estrogen therapy on endometrial fibrosis in IUA rats. Our findings revealed that IUA rats exhibited severe endometrial damage, heightened inflammatory responses, significant collagen deposition, and imbalances in various inflammatory and growth factors. However, these pathological changes were markedly improved following combined rrGH and estrogen treatment. Additionally, the endometrial microbial diversity in IUA rats was significantly reduced, and the combined therapy effectively promoted its restoration. Biochemical serum analysis showed that the combined therapy upregulated key reproductive hormone levels. Notably, the combined application of rrGH and estrogen partially restored GH receptor levels. TGF-β1, MMP9, and β-catenin were upregulated in the endometria of IUA rats, while the p-smad3/smad3 ratio was downregulated, and these key indicators were reversed after combined therapy. Furthermore, antibiotic treatment weakened the effects of combined therapy, indicating the role of endometrial microbiota in IUA. Molecular docking results revealed a high affinity between β-catenin and differential metabolites such as citraconic acid, suggesting their potential importance in regulating the β-catenin signaling pathway. In a TGF-β1-induced IUA cell model, we found that TGF-β1 treatment upregulated fibrosis-related protein expression but decreased β-catenin protein levels and stability. Citraconic acid intervention enhanced the effects of TGF-β1, while β-catenin overexpression inhibited these changes. In summary, the combined therapy targeting the β-catenin pathway through citraconic acid regulation alleviated endometrial fibrosis, offering a new approach to treating IUA.IMPORTANCEIntrauterine adhesions (IUAs) are an important endometrial disease. Our study highlights the importance of the combination of recombinant rat growth hormone (rrGH) and estrogen in ameliorating endometrial damage and fibrosis, as well as promoting endometrial regeneration in IUA rats. In addition, our study emphasizes their important role in ameliorating microecological disturbances in the intrauterine environment and regulating serum metabolism. Our experiments also revealed for the first time that the combination of rrGH and estrogen may modulate endometrial microbes or influence the progression of IUA by promoting β-catenin expression, which is important for understanding the treatment of IUA disease. Our study provides new and important insights into the understanding and treatment of IUA disease.
Keywords: citraconic acid; endometrial microbiota; estrogen; growth hormone; intrauterine adhesions; β-catenin pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Human amnion mesenchymal stem cells promote endometrial repair via paracrine, preferentially than transdifferentiation.Cell Commun Signal. 2024 May 31;22(1):301. doi: 10.1186/s12964-024-01656-0. Cell Commun Signal. 2024. PMID: 38822356 Free PMC article.
-
Restoration of functional endometrium in an intrauterine adhesion rat model with endometrial stromal cells transplantation.Stem Cell Res Ther. 2024 Jun 21;15(1):181. doi: 10.1186/s13287-024-03788-z. Stem Cell Res Ther. 2024. PMID: 38902788 Free PMC article.
-
Exploring the application value of ultrasound in animal studies of stem cell therapy for intrauterine adhesions.Sci Rep. 2025 Aug 11;15(1):29319. doi: 10.1038/s41598-025-14996-9. Sci Rep. 2025. PMID: 40789897 Free PMC article.
-
Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review.J Minim Invasive Gynecol. 2014 Jan-Feb;21(1):44-54. doi: 10.1016/j.jmig.2013.07.018. Epub 2013 Aug 9. J Minim Invasive Gynecol. 2014. PMID: 23933351
-
Mechanistic insights into intrauterine adhesions.Semin Immunopathol. 2024 Nov 29;47(1):3. doi: 10.1007/s00281-024-01030-9. Semin Immunopathol. 2024. PMID: 39613882 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
