Alnustone Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease by Facilitating Mitochondrial Fatty Acid β-Oxidation via Targeting Calmodulin
- PMID: 40470949
- PMCID: PMC12376512
- DOI: 10.1002/advs.202411984
Alnustone Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease by Facilitating Mitochondrial Fatty Acid β-Oxidation via Targeting Calmodulin
Abstract
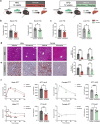
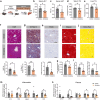
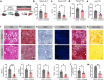
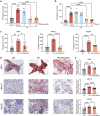
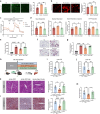
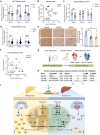
Metabolic dysfunction-associated steatotic liver disease (MASLD), including its more severe manifestation metabolic dysfunction-associated steatohepatitis (MASH), poses global public health threats with limited therapeutics. Here, the role of alnustone is explored, a natural compound derived from the traditional Chinese herb Alpinia katsumadai Hayata, in the treatment of MASLD and MASH. It is shown that alnustone administration potently reduces serum triacylglycerol levels, reverses liver steatosis, and alleviates insulin resistance in both male and female MASLD mice. It also effectively ameliorates established fibrosis in MASH mice without any side effects. Mechanistically, hepatic lipidome profiling and energy metabolic assays reveal that alnustone facilitates mitochondrial fatty acid β-oxidation. Employing limited proteolysis-mass spectrometry (LiP-SMap) and further validation, calmodulin is identified as a direct molecular target of alnustone. Alnustone interacts with the Ca2+-binding site of calmodulin, leading to increased cytosolic and mitochondrial Ca2+ levels and enhanced mitochondrial function, whereas liver-specific calmodulin knockdown abrogates alnustone's therapeutic effects. Moreover, calmodulin is downregulated in human livers of patients with MASLD and MASH, and is genetically associated with reduced MASLD risk. These findings establish alnustone as a promising natural compound and highlight calmodulin as a target for treating MASLD.
Keywords: alnustone; calmodulin; fatty acid β‐oxidation; metabolic dysfunction‐associated steatohepatitis; metabolic dysfunction‐associated steatotic liver disease.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rinella M. E., Lazarus J. V., Ratziu V., Francque S. M., Sanyal A. J., Kanwal F., Romero D., Abdelmalek M. F., Anstee Q. M., Arab J. P., Arrese M., Bataller R., Beuers U., Boursier J., Bugianesi E., Byrne C. D., Castro Narro G. E., Chowdhury A., Cortez‐Pinto H., Cryer D. R., Cusi K., El‐Kassas M., Klein S., Eskridge W., Fan J., Gawrieh S., Guy C. D., Harrison S. A., Kim S. U., Koot B. G., et al., Hepatology 2023, 78, 1966.
-
- Wong V. W., Ekstedt M., Wong G. L., Hagström H., J Hepatol 2023, 79, 842. - PubMed
-
- Schuster S., Cabrera D., Arrese M., Feldstein A. E., Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349. - PubMed
MeSH terms
Substances
Grants and funding
- 32588201/National Natural Science Foundation of China
- 82421004/National Natural Science Foundation of China
- 82371644/National Natural Science Foundation of China
- 82100839/National Natural Science Foundation of China
- 2021-I2M-5-001/CAMS Innovation Fund for Medical Sciences
- 2024CXPT087/Shandong Provincial Key Research and Development Program
- 2024BEG02019/Ningxia Hui Autonomous Region Key Research and Developmental Program
- ZR2020QH083/Shandong Provincial Natural Science Foundation
- 2023KJ021/Fundamental Research Funds of Shandong University
- 2023QNTD004/Fundamental Research Funds of Shandong University
- ts20190988/Taishan Scholar Foundation of Shandong Province
- tsqn202312387/Taishan Scholar Foundation of Shandong Province
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
