Taperin bundles F-actin at stereocilia pivot points enabling optimal lifelong mechanosensitivity
- PMID: 40471101
- PMCID: PMC12139522
- DOI: 10.1083/jcb.202408026
Taperin bundles F-actin at stereocilia pivot points enabling optimal lifelong mechanosensitivity
Abstract
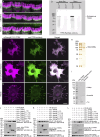
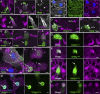
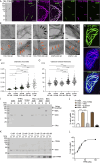
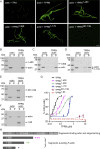
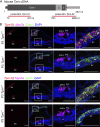
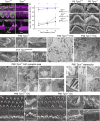
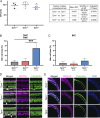
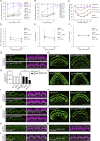
Stereocilia are rod-like mechanosensory projections consisting of unidirectionally oriented actin filaments that extend into the inner ear hair cell cytoskeleton, forming dense rootlets. Taperin (TPRN) localizes to the narrowed-down base of stereocilia, where they pivot in response to sound and gravity. We show that TPRN-deficient mice have progressive deafness characterized by gradual asynchronous retraction and fusion of outer and inner hair cell stereocilia, followed by synaptic abnormalities. Stereocilia that lack TPRN develop warped rootlets with gradual loss of TRIOBP-5 and ANKRD24 from mechanosensory rows starting postnatally. In contrast, TPRN overexpression causes excessive F-actin bundling, extra rows, and over-elongation of stereocilia during development. Purified full-length mouse TPRN cross-links F-actin into bendable bundles reflecting in vivo data. This F-actin-bundling ability is attributed to the TPRN N-terminal region. TPRN interacts with the membrane receptor PTPRQ, connecting the F-actin core to the plasma membrane, stabilizing stereocilia. Thus, TPRN is a specialized F-actin bundler strategically located to augment stereocilia rootlet formation and their pivot point flexibility for sustained sound-induced deflections.
© 2025 Belyantseva et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures






References
MeSH terms
Substances
Grants and funding
- 1542174/Division of Electrical, Communications and Cyber Systems
- R01DC019054/NH/NIH HHS/United States
- DC000039/DC/NIDCD NIH HHS/United States
- R01DC014658/NH/NIH HHS/United States
- R01 DC019054/DC/NIDCD NIH HHS/United States
- S10OD025130/NH/NIH HHS/United States
- R01 DC018785/DC/NIDCD NIH HHS/United States
- R01DC017147/NH/NIH HHS/United States
- R01 DC017147/DC/NIDCD NIH HHS/United States
- R01 DC015495/DC/NIDCD NIH HHS/United States
- R01 DC014658/DC/NIDCD NIH HHS/United States
- DC-000080/DC/NIDCD NIH HHS/United States
- S10 OD025130/OD/NIH HHS/United States
- ZIC DC000080/ImNIH/Intramural NIH HHS/United States
- T32 DC000039/DC/NIDCD NIH HHS/United States
- University of Kentucky Electron Microscopy Center
- Z01 DC000039/ImNIH/Intramural NIH HHS/United States
- Intramural Research Program
- National Science Foundation
- R01DC018785/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

