Extended disability leave and related costs among employed patients with versus without graft-versus-host disease following hematopoietic stem cell transplantation
- PMID: 40471352
- PMCID: PMC12141405
- DOI: 10.1007/s00520-025-09561-z
Extended disability leave and related costs among employed patients with versus without graft-versus-host disease following hematopoietic stem cell transplantation
Abstract
Purpose: Post-hematopoietic stem cell transplantation (HSCT) graft-versus-host disease (GVHD) is associated with considerable healthcare costs, and impaired productivity may represent an additional financial burden. This analysis quantified GVHD-associated indirect costs of productivity loss by comparing work-related disability leave claims between patients with versus without GVHD.
Methods: Patients with claims for HSCT in the IBM® MarketScan Commercial Database and the Health and Productivity Management Database between 1/1/09-12/31/19 and associated short- or long-term disability claims were included.
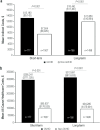
Results: Of 354 patients with GVHD and 2629 patients without GVHD included in the analysis, a significantly greater percentage of patients with versus without GVHD had claims for short-term disability (GVHD, 43.5%; non-GVHD, 29.2%; P < 0.001), long-term disability (GVHD, 19.9%; non-GVHD, 6.3%; P < 0.001) or a combination of both (GVHD, 53.5%; non-GVHD, 30.5%; P < 0.001). Disability leaves were, on average, longer for patients with GVHD versus without GVHD (short-term, 103 vs 59 days, P < 0.001; long-term, 120 vs 92 days, P < 0.001). Mean indirect costs of workdays lost due to disability leave were significantly higher among those with versus without GVHD (short-term, $13,180 vs $7504, P < 0.001; long-term, $15,441 vs $11,850, P < 0.001). Mean all-cause healthcare costs were significantly higher among those with versus without GVHD (short-term leave: $295,241 vs $95,937, P < 0.001; long-term leave: $312,691 vs $94,285, P < 0.001).
Conclusions: Most full-time employed patients with GVHD took disability leave, accounting for approximately half (127/261) of their total workdays, incurring higher indirect costs than patients without GVHD. Additionally, all-cause healthcare costs were threefold higher in patients with versus without GVHD.
Keywords: Costs; Disability; Graft-versus-host disease; Hematopoietic stem cell transplantation; Productivity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Ethics approval was not applicable. Consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: JY, VB, and JG are employees and stockholders of Incyte Corporation. ET is an employee of Merative, the company commissioned by Incyte Corporation to perform this analysis. KJ-P was an employee of Merative at the time of the study.
Figures

References
-
- Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, Giralt SA, LeMaistre CF, Marks DI, Omel JL, Orchard PJ, Palmer J, Saber W, Veys PA, Carpenter PA, Hamadani M (2020) Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant 26:1247–1256. 10.1016/j.bbmt.2020.03.002 - DOI - PubMed
-
- Passweg JR, Baldomero H, Basak GW, Chabannon C, Corbacioglu S, Duarte R, Kuball J, Lankester A, Montoto S, de Latour RP, Snowden JA, Styczynski J, Yakoub-Agha I, Arat M, Mohty M, Kröger N, European Society for Blood and Marrow Transplantation (EBMT) (2019) The EBMT activity survey report 2017: a focus on allogeneic HCT for nonmalignant indications and on the use of non-HCT cell therapies. Bone Marrow Transplant 54:1575–1585. 10.1038/s41409-019-0465-9 - PMC - PubMed
-
- Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, Corbacioglu S, Dreger P, Dufour C, Gennery AR, Kuball J, Lankester AC, Lanza F, Montoto S, Nagler A, Peffault de Latour R, Snowden JA, Styczynski J, Yakoub-Agha I, Kröger N, Mohty M for the European Society for Blood and Marrow Transplantation (EBMT) (2019) Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant 54:1525–1552. 10.1038/s41409-019-0516-2 - PubMed
-
- Axt L, Naumann A, Toennies J, Haen SP, Vogel W, Schneidawind D, Wirths S, Moehle R, Faul C, Kanz L, Axt S, Bethge WA (2019) Retrospective single center analysis of outcome, risk factors and therapy in steroid refractory graft-versus-host disease after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 54:1805–1814. 10.1038/s41409-019-0544-y - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

