Real-World Safety and Effectiveness of Vosoritide in Achondroplasia: Results from a Single Center in Portugal
- PMID: 40471380
- PMCID: PMC12313777
- DOI: 10.1007/s12325-025-03223-6
Real-World Safety and Effectiveness of Vosoritide in Achondroplasia: Results from a Single Center in Portugal
Abstract
Introduction: Achondroplasia, the most common skeletal dysplasia, is caused by autosomal dominant gain-of-function pathogenic variants in the fibroblast growth factor receptor 3 (FGFR3) gene. Vosoritide, a C-type natriuretic peptide analog, is a first-in-class targeted treatment for achondroplasia that counteracts overactive FGFR3 signaling to stimulate endochondral bone growth. This retrospective cohort study evaluated growth, safety, and treatment compliance in children with achondroplasia receiving vosoritide under an early access program in Portugal.
Methods: Twenty-seven children aged 2-14 years with a genetically confirmed diagnosis of achondroplasia were treated with vosoritide at a single Portuguese center for at least 6 months between January 2022 and June 2024. The analysis included children with severe achondroplasia-associated complications. Anthropometric measurements collected to characterize the effect of vosoritide on growth included height standard deviation score (SDS) and annualized growth velocity (AGV). Student's t test was used for statistical comparisons. Safety and tolerability endpoints included adverse drug reactions and treatment adherence.
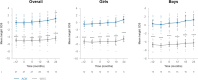
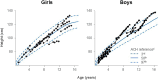
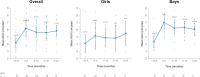
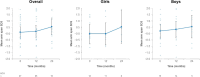
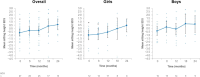
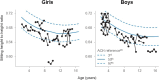
Results: In total, 15 children completed at least 24 months of treatment. After 24 months of treatment, mean variation in height SDS increased from baseline by + 0.95 SD (P ≤ 0.0001), referenced to an untreated achondroplasia-specific population, and + 0.56 SD (P ≤ 0.0001) relative to children of average stature. Additionally, mean AGV from baseline was 5.87 cm/year (95% confidence interval 5.14-6.60), resulting in a significant increase of + 1.62 cm/year (P ≤ 0.0001). Injection site reactions were the most common adverse drug reaction observed (n = 14); no serious adverse drug reactions were reported. There were no discontinuations due to adverse drug reactions.
Conclusion: Vosoritide showed long-term effectiveness in a real-world Portuguese population of patients with achondroplasia. Vosoritide was also well tolerated, and patients showed good adherence to treatment. These findings were consistent with the outcomes of clinical trials and existing real-world experience.
Keywords: Achondroplasia; Compliance; Growth; Proportionality; Real-world experience; Safety; Vosoritide.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Inês Rua has received honoraria for lectures and/or travel support from BioMarin and Ascendis; and has been an investigator in observational studies and/or clinical trials for Pfizer, Ascendis, BioMarin, and QED. Isabel Silva has received travel support from BioMarin; and has been an investigator in observational studies for BioMarin. Upon completion of the manuscript, Isabel Silva has changed affiliation to Unidade Local de Saúde de São José, Lisbon, Portugal. Christoph Beger has received support for attending meetings and/or travel from BioMarin. Cristina Gomes has received honoraria for lectures and/or travel support from BioMarin; and has been research nurse in observational studies or clinical trials for Pfizer, Ascendis, BioMarin, and QED. Maria J. Pais has been research nurse in observational studies and/or clinical trials for Pfizer, BioMarin, and QED. Alice Mirante has received honoraria for lectures, advisory boards, and/or travel support from BioMarin; and has been an investigator in observational studies and/or clinical trials for Pfizer, Ascendis, and BioMarin. Sérgio B. Sousa has received honoraria for lectures, advisory boards, and/or travel support from BioMarin, Ascendis, and Kiowa Kirin; and has been an investigator in observational studies and/or clinical trials for Pfizer, Ascendis, BioMarin, and QED. Ethical Approval: The study was conducted in accordance with the Declaration of Helsinki, and approval from Unidade Local de Saúde de Coimbra ethics committee was obtained (ref. OBS.SF.140-2022; no. 490/CE; 1 August, 2024). Written consent was obtained for the use of any identifying information.
Figures

References
-
- Savarirayan R, Ireland P, Irving M, et al. International Consensus Statement on the diagnosis, multidisciplinary management and lifelong care of individuals with achondroplasia. Nat Rev Endocrinol. 2022;18:173–89. - PubMed
-
- Almeida MR, Campos-Xavier AB, Medeira A, et al. Clinical and molecular diagnosis of the skeletal dysplasias associated with mutations in the gene encoding fibroblast growth factor receptor 3 (FGFR3) in Portugal. Clin Genet. 2009;75:150–6. - PubMed
-
- Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet. 2007;370:162–72. - PubMed
-
- Merker A, Neumeyer L, Hertel NT, et al. Growth in achondroplasia: development of height, weight, head circumference, and body mass index in a European cohort. Am J Med Genet A. 2018;176:1723–34. - PubMed
-
- del Pino M, Fano V, Lejarraga H. Growth references for height, weight, and head circumference for Argentine children with achondroplasia. Eur J Pediatr. 2011;170:453–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

