Meningeal Embolization for Preventing Chronic Subdural Hematoma Recurrence After Surgery: The EMPROTECT Randomized Clinical Trial
- PMID: 40471557
- PMCID: PMC12142473
- DOI: 10.1001/jama.2025.7583
Meningeal Embolization for Preventing Chronic Subdural Hematoma Recurrence After Surgery: The EMPROTECT Randomized Clinical Trial
Abstract
Importance: Middle meningeal artery (MMA) embolization has been proposed as a potential treatment for chronic subdural hematoma (CSDH).
Objective: To assess the efficacy of MMA embolization in reducing the risk of CSDH recurrence at 6 months compared with standard care in patients who underwent an operation and were at high risk of CSDH recurrence.
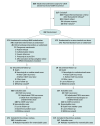
Design, setting, and participants: Multicenter, open-label, randomized clinical trial with blinded end point assessment. Patients who underwent an operation for CSDH recurrence or a first CSDH episode at high risk of recurrence were recruited from July 2020 to March 2023 in 12 French neurosurgical or comprehensive neurosurgical and interventional neuroradiology centers. Last follow-up took place on November 2, 2023.
Intervention: Participants were randomized 1:1 to undergo MMA embolization with microparticles within 7 days of surgery (171 patients, intervention group) or standard medical care alone (171 patients, control group).
Main outcomes and measures: The primary end point was the rate of CSDH recurrence at 6 months assessed by an independent, blinded adjudication committee. There were 5 secondary end points, including rates of repeat surgery for homolateral CSDH recurrence during the 6-month follow-up period and embolization procedure-related complications.
Results: Among 342 randomized patients (median [IQR] age, 77 [68-83] years; 274 [80.1%] male), 308 (90.1%) completed the trial. The primary end point was observed in 24 of 162 (14.8%) and 33 of 157 (21.0%) patients in the intervention and control groups, respectively (after imputation: odds ratio, 0.64 [95% CI, 0.36-1.14]; adjusted absolute difference, -6% [95% CI, -14% to 2%]; P = .13). The groups did not significantly differ in any of the secondary end points. Repeat surgery was performed in 7 of 162 (4.3%) and 13 of 157 (8.3%) patients in the intervention and control groups (P = .14), respectively. Minor and major embolization procedure-related complications occurred in 3 of 171 (1.8%) and 1 of 171 (0.6%) patients, respectively.
Conclusions and relevance: In this randomized clinical trial, among patients who underwent an operation for CSDH recurrence or a first CSDH episode at high risk of recurrence, MMA embolization did not lead to a significantly lower rate of recurrence at 6 months compared with standard medical care alone. However, the magnitude of the effect estimate is consistent with other recent trials, including some that demonstrated the benefit of MMA embolization with nonadhesive liquid embolic agents, and these findings considered together may inform future studies and potential use of this therapeutic approach for CSDH management.
Trial registration: ClinicalTrials.gov Identifier: NCT04372147.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

