The microRNA miR-30a blocks adipose tissue fibrosis accumulation in obesity
- PMID: 40471675
- PMCID: PMC12321386
- DOI: 10.1172/JCI175566
The microRNA miR-30a blocks adipose tissue fibrosis accumulation in obesity
Abstract
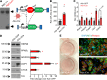
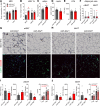
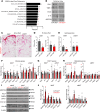
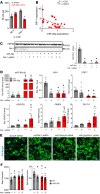
White adipose tissue (WAT) fibrosis occurring in obesity contributes to the inflammatory and metabolic comorbidities of insulin resistance and type 2 diabetes, yet the mechanisms involved remain poorly understood. Here, we report a role for the broadly conserved miRNA miR-30a as a regulator of WAT fibrosis and systemic glucose metabolism. Mice modified to express miR-30a at elevated levels in adipose tissues maintain insulin sensitivity coupled with reduced fatty liver disease when fed a high-fat diet. These effects were attributable to cell-autonomous functions of miR-30a that potently increase expression of adipocyte-specific genes. Proteomic screening revealed miR-30a limits profibrotic programs in subcutaneous WAT, at least in part, by repressing PAI-1, a dominant regulator of fibrinolysis and biomarker of insulin resistance. Conversely, mouse adipocytes lacking miR-30a exhibited greater expression of fibrosis markers with disrupted cellular metabolism. Lastly, miR-30a expression negatively correlates with PAI-1 levels in subcutaneous WAT from people with obesity, further supporting an antifibrotic role for miR-30a. Together, these findings uncover miR-30a as a critical regulator of adipose tissue fibrosis that predicts metabolically healthy obesity in people and mice.
Keywords: Adipose tissue; Cell biology; Fibrosis; Metabolism; Noncoding RNAs.
Conflict of interest statement
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

