Remote early detection of SARS-CoV-2 infections using a wearable-based algorithm: Results from the COVID-RED study, a prospective randomised single-blinded crossover trial
- PMID: 40471995
- PMCID: PMC12140236
- DOI: 10.1371/journal.pone.0325116
Remote early detection of SARS-CoV-2 infections using a wearable-based algorithm: Results from the COVID-RED study, a prospective randomised single-blinded crossover trial
Abstract
Background: Rapid and early detection of SARS-CoV-2 infections, especially during the pre- or asymptomatic phase, could aid in reducing virus spread. Physiological parameters measured by wearable devices can be efficiently analysed to provide early detection of infections. The COVID-19 Remote Early Detection (COVID-RED) trial investigated the use of a wearable device (Ava bracelet) for improved early detection of SARS-CoV-2 infections in real-time.
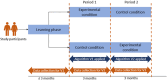
Trial design: Prospective, single-blinded, two-period, two-sequence, randomised controlled crossover trial.
Methods: Subjects wore a medical device and synced it with a mobile application in which they also reported symptoms. Subjects in the experimental condition received real-time infection indications based on an algorithm using both wearable device and self-reported symptom data, while subjects in the control arm received indications based on daily symptom-reporting only. Subjects were asked to get tested for SARS-CoV-2 when receiving an app-generated alert, and additionally underwent periodic SARS-CoV-2 serology testing. The overall and early detection performance of both algorithms was evaluated and compared using metrics such as sensitivity and specificity.
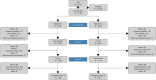
Results: A total of 17,825 subjects were randomised within the study. Subjects in the experimental condition received an alert significantly earlier than those in the control condition (median of 0 versus 7 days before a positive SARS-CoV-2 test). The experimental algorithm achieved high sensitivity (93.8-99.2%) but low specificity (0.8-4.2%) when detecting infections during a specified period, while the control algorithm achieved more moderate sensitivity (43.3-46.4%) and specificity (66.4-65.0%). When detecting infection on a given day, the experimental algorithm also achieved higher sensitivity compared to the control algorithm (45-52% versus 28-33%), but much lower specificity (38-50% versus 93-97%).
Conclusions: Our findings highlight the potential role of wearable devices in early detection of SARS-CoV-2. The experimental algorithm overestimated infections, but future iterations could finetune the algorithm to improve specificity and enable it to differentiate between respiratory illnesses.
Trial registration: Netherlands Trial Register number NL9320.
Copyright: © 2025 Zwiers et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have read the journal’s policy and have the following competing interests: Laura Zwiers, Marcel van Willigen, Jon Bouwman and Diederick Grobbee are current employees of Julius Clinical BV. Timo Brakenhoff, Brianna Goodale and Duco Veen are former employees of Julius Clinical BV. Billy Franks is a former employee of Julius Clinical BV and now an employee of Haleon. Brianna Goodale, Vladimir Kovacevic, Andjela Markovic and Maureen Cronin are past employees of Ava AG. Marianna Mitratza is a current employee of P95 CVBA. Lorenz Risch and Martin Risch are current employees and key shareholders of Dr Risch Medical Laboratory. Kirsten Grossman and Ornella Weideli are current or former employees of Dr Risch Medical Laboratory. David Conen has received consulting fees from Roche Diagnostics, outside of the current work. There are no patents, products in development or marketed products associated with this research to declare. These competing interests do not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- WHO Coronavirus (COVID-19) Dashboard. [cited 2024 Apr 17]. Available from: https://data.who.int/dashboards/covid19/cases
-
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci AM, et al.. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17(9):e1003346. doi: 10.1371/journal.pmed.1003346 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous