Indirect comparison of epcoritamab vs chemoimmunotherapy, mosunetuzumab, or odronextamab in follicular lymphoma
- PMID: 40472301
- PMCID: PMC12309599
- DOI: 10.1182/bloodadvances.2024015274
Indirect comparison of epcoritamab vs chemoimmunotherapy, mosunetuzumab, or odronextamab in follicular lymphoma
Abstract
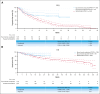
This matching-adjusted indirect comparison evaluated the efficacy of epcoritamab vs standard of care (SOC), mosunetuzumab, or odronextamab, and assessed safety vs mosunetuzumab and odronextamab. Individual patient-level data from the EPCORE NHL-1 follicular lymphoma (FL) cohort for epcoritamab were used with pooled data from SCHOLAR-5 for SOC (mostly chemoimmunotherapy [CIT]), and aggregate data from GO29781 for mosunetuzumab and ELM-2 for odronextamab. Trial populations were match-adjusted using propensity score weights for key baseline characteristics. Compared with SOC/CIT, epcoritamab provided significantly higher response rates (overall response rate [ORR], 90.9% vs 56.8%; P < .001; complete response [CR] rate, 73.7% vs 32.0%; P < .001). Epcoritamab showed numerically higher ORR (90.9% vs 80.0%; P = .067) and CR rate (72.8% vs 60.0%; P = .159) vs mosunetuzumab. Epcoritamab provided significantly higher ORR (91.5% vs 80.5%; P = .026) and numerically lower CR rate (67.5% vs 73.4%; P = .428) vs odronextamab. Epcoritamab did not have any grade ≥3 cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS; any grade) events compared with CRS (grade ≥3) in 2.2% and 3.9% and ICANS in 4.4% and 0.8% of patients treated with mosunetuzumab and odronextamab, respectively (P < .001). In addition to being a convenient subcutaneous option, epcoritamab showed significantly superior response rates and survival outcomes vs SOC/CIT among patients with relapsed or refractory FL after ≥2 systemic therapies. Epcoritamab also exhibited clinically relevant, numerically higher ORRs and demonstrated improved safety for CRS (grade ≥3) and ICANS vs mosunetuzumab or odronextamab. These trials were registered at www.ClinicalTrials.gov as #NCT03625037, #NCT02500407, and #NCT03888105.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.V.D. reports consulting fees from AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Genentech, Genmab, Incyte, Janssen, Lilly Oncology, MEI Pharma, Merck, MorphoSys, and Nurix, and research funding from AbbVie, AstraZeneca, Bayer Oncology, Bristol Myers Squibb, Cyclacel, MEI Pharma, and Nurix. S.K.T. reports research funding from Genentech, Genmab, Ipsen, and ADC Therapeutics; consultancy for AbbVie; and advisory board participation for Ipsen. K.L. reports consulting or advisory roles for AbbVie, Roche, Genmab, Kite/Gilead, BeiGene, Bristol Myers Squibb, and Celgene; honoraria from AbbVie, Celgene, Hartley Taylor, and Roche; research grants from Takeda, Roche, Genmab, and AbbVie; and support to attend meetings from Celgene and Genmab. K.C. and V.C. are employees of OPEN Health, which received funding support from Genmab to conduct the research. A.M., S.B.C., F.R.N., F.M.G., Z.D., and E.F. are employees of and hold stock in Genmab. A.C. and D.H. are former employees of Genmab. A.W. and A.A. are employees of and hold stock in AbbVie. A.S. reports consultancy for Takeda, Bristol Myers Squibb/Celgene, Novartis, Janssen, Merck Sharp & Dohme, Amgen, GlaxoSmithKline, Sanofi, Kite, and Mundipharma; honoraria from Takeda, Bristol Myers Squibb/Celgene, Novartis, Janssen, Merck Sharp & Dohme, Amgen, GlaxoSmithKline, Sanofi, and Kite; membership on an entity’s board of directors or advisory committees for Takeda, Bristol Myers Squibb/Celgene, Novartis, Janssen, Amgen, Bluebird, Sanofi, and Kite; travel expenses from Takeda, Bristol Myers Squibb/Celgene, and Roche; research funding from Takeda; and speakers' bureau participation for Takeda, Bristol Myers Squibb/Celgene, Novartis, Janssen, Merck Sharp & Dohme, Amgen, GlaxoSmithKline, Sanofi, and Kite.
Figures




References
-
- Oerlemans S, Issa DE, van den Broek EC, et al. Impact of therapy and disease-related symptoms on health-related quality of life in patients with follicular lymphoma: results of the population-based PHAROS-registry. Eur J Haematol. 2014;93(3):229–238. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

