Prospective study of immunogenicity to SARS-CoV-2 booster vaccines in multiple myeloma and Waldenström macroglobulinemia
- PMID: 40472329
- PMCID: PMC12452669
- DOI: 10.1182/bloodadvances.2025016513
Prospective study of immunogenicity to SARS-CoV-2 booster vaccines in multiple myeloma and Waldenström macroglobulinemia
Abstract
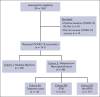
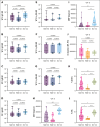
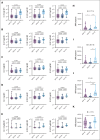
Patients with multiple myeloma (MM) and Waldenström macroglobulinemia (WM) have increased risk of severe coronavirus disease 2019. Impaired humoral responses to the primary vaccination series against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been reported in patients with MM and WM, but the impact of booster vaccinations and functional status of the elicited antibodies are unknown. We performed a prospective cohort study investigating SARS-CoV-2 vaccination in patients with MM (n = 93) and WM (n = 48). The primary end point was effective immune response (EIR) at 28 days after the primary vaccination series (ie, anti-spike SARS-CoV-2 antibody titer >250 U/mL). The EIR rate after the primary vaccination series was 47% and 25% in patients with MM and WM, respectively. After the first and second booster vaccinations, the EIR rates increased to 84% and 91% in patients with MM and 60% and 89% in those with WM, respectively. Factors associated with lower EIR in patients with MM were hypogammaglobulinemia, lymphopenia, and treatment with anti-CD38 monoclonal antibody or corticosteroid. In patients with WM, treatment with a Bruton tyrosine kinase inhibitor or rituximab was associated with a lower EIR, whereas asymptomatic and treatment-naïve patients had a higher EIR. Functional profiling identified reduced antibody-dependent cellular phagocytosis, neutrophil phagocytosis, and natural killer cell activity against wild-type and variant SARS-CoV-2 (Alpha, Beta, and Gamma) in patients with MM and WM compared to healthy donors. Our data demonstrate that although patients with MM and WM have impaired humoral responses to the primary SARS-CoV-2 vaccination series, repeated booster vaccines significantly improve immunogenicity. This trial was registered at www.ClinicalTrials.gov as #NCT04830046.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.R.B. received honoraria/research funding from Adaptive Biotechnologies, BeiGene, CSL Behring, Genzyme, Karyopharm, Pharmacyclics, and Sanofi. J.J.C. received honoraria from AbbVie, AstraZeneca, BeiGene, Cellectar, Johnson & Johnson (J&J), Kite, Loxo, and Pharmacyclics; and research funding from AbbVie, AstraZeneca, BeiGene, Cellectar, Loxo, and Pharmacyclics. S.P.T. received honoraria/research funding from ADC Therapeutics, AstraZeneca, and BeiGene. S.R.S. received honoraria/research funding from ADC Therapeutics, AstraZeneca, and BeiGene. P.G.R. received honoraria/research funding from Celgene/Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), Jazz Pharmaceuticals, Karyopharm, Oncopeptides, Regeneron, and Sanofi. A.J.Y. reports consulting for AbbVie, Adaptive Biotechnologies, Amgen, BMS, Celgene, GSK, J&J, Karyopharm, Oncopeptides, Pfizer, Prothena, Regeneron, Sanofi, Sebia, and Takeda; and research funding to institution from Amgen, BMS, GSK, J&J, and Sanofi. M.L. received honoraria from Genmab, Sanofi, MJH Life Sciences, Genentech, Center for Business Models, and C4X Discovery. C.S.M. has served on the scientific advisory board of Adicet Bio; reports consultancy/honararia form Genentech, Nerviano, Secura Bio, and Oncopeptides; and research funding from EMGD Serono, Karyopharm, Sanofi, Nurix, BMS, H3 Biomedicine/Eisai, Springworks, Abcuro, Novartis, and Opna Bio. The remaining authors declare no competing financial interests.
Figures

References
-
- Lund SH, Hultcrantz M, Goldin L, et al. Patterns of infectious morbidity in patients with Waldenström’s macroglobulinaemia/lymphoplasmacytic lymphoma: a population-based study. Blood. 2014;124(21):3350.
-
- Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 2009;49(8):1211–1225. - PubMed
-
- Raje NS, Anaissie E, Kumar SK, et al. Consensus guidelines and recommendations for infection prevention in multiple myeloma: a report from the International Myeloma Working Group. Lancet Haematol. 2022;9(2):e143–e161. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

