Synthesis of Isoquinoline N-Oxides via Hypervalent Iodine-Mediated Oxidative Cyclization of Ketoximes with Alkenes
- PMID: 40472394
- PMCID: PMC12186619
- DOI: 10.1021/acs.joc.5c00488
Synthesis of Isoquinoline N-Oxides via Hypervalent Iodine-Mediated Oxidative Cyclization of Ketoximes with Alkenes
Abstract
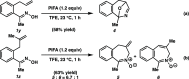
Reported herein is the development of an intramolecular oxidative cyclization of ketoximes with alkenes for the preparation of isoquinoline N-oxides. The reaction, which utilizes phenyliodine bis(trifluoroacetate) (PIFA) as an oxidant and 2,2,2-trifluoroethanol (TFE) as a solvent, proceeds to afford various N-heterocyclic products, including aryl/heteroaryl-fused pyridine N-oxides, isoindole N-oxides, and 2-benzazepine derivatives. Preliminary experimental and computational mechanistic studies suggest that the ionic pathway is the primary mechanism. The synthetic utility of the developed method was highlighted via several product transformations.
Figures





References
-
- Dembitsky V. M., Gloriozova T. A., Poroikov V. V.. Naturally Occurring Plant Isoquinoline N-Oxide Alkaloids: Their Pharmacological and SAR Activities. Phytomedicine. 2015;22:183–202. doi: 10.1016/j.phymed.2014.11.002. - DOI - PubMed
- Bull J. A., Mousseau J. J., Pelletier G., Charette A. B.. Synthesis of Pyridine and Dihydropyridine Derivatives by Regio- and Stereoselective Addition to N-Activated Pyridines. Chem. Rev. 2012;112:2642–2713. doi: 10.1021/cr200251d. - DOI - PubMed
- Escolano M., Gaviña D., Alzuet-Piña G., Díaz-Oltra S., Sánchez-Roselló M., del Pozo C.. Recent Strategies in the Nucleophilic Dearomatization of Pyridines, Quinolines, and Isoquinolines. Chem. Rev. 2024;124:1122–1246. doi: 10.1021/acs.chemrev.3c00625. - DOI - PMC - PubMed
-
- Youssif S.. Recent Trends in the Chemistry of Pyridine N-Oxide. ARKIVOC. 2001;2001:242–268. doi: 10.3998/ark.5550190.0002.116. - DOI
-
- Li B., Jiao P., Zhong H., Huang J.. Isoquinoline N-Oxide Synthesis under Pd-Catalysed C–H Activation/Annulation Processes. Synlett. 2013;24:2431–2436. doi: 10.1055/s-0033-1339671. - DOI
- Liu J., Yang Z., Long H., Xian J., Zheng L., Liu Z.-Q.. Oxidative Cyclization of Oximes with Propargyl Alcohols toward Functionalized Isoquinoline N-Oxides Synthesis. Asian J. Org. Chem. 2024;13:e202300562. doi: 10.1002/ajoc.202300562. - DOI
- Li Y., Fang F., Zhou J., Li J., Wang R., Liu H., Zhou Y.. Rhodium-Catalyzed C–H Activation/Annulation Cascade of Aryl Oximes and Propargyl Alcohols to Isoquinoline N-Oxides. Adv. Synth. Catal. 2021;363:3305–3310. doi: 10.1002/adsc.202100239. - DOI
- Shi Z., Koester D. C., Boultadakis-Arapinis M., Glorius F.. Rh(III)-Catalyzed Synthesis of Multisubstituted Isoquinoline and Pyridine N-Oxides from Oximes and Diazo Compounds. J. Am. Chem. Soc. 2013;135:12204–12207. doi: 10.1021/ja406338r. - DOI - PubMed
- Phatake R. S., Patel P., Ramana C. V.. Ir(III)-Catalyzed Synthesis of Isoquinoline N-Oxides from Aryloxime and α-Diazocarbonyl Compounds. Org. Lett. 2016;18:292–295. doi: 10.1021/acs.orglett.5b03462. - DOI - PubMed
-
- Yeom H.-S., Kim S., Shin S.. Silver(I)-Catalyzed Direct Route to Isoquinoline-N-Oxides. Synlett. 2008;2008:924–928. doi: 10.1055/s-2008-1042936. - DOI
- Huo Z., Tomeba H., Yamamoto Y.. Iodine-Mediated Electrophilic Cyclization of 2-Alkynylbenzaldoximes Leading to the Formation of Iodoisoquinoline N-Oxides. Tetrahedron Lett. 2008;49:5531–5533. doi: 10.1016/j.tetlet.2008.07.061. - DOI
- Araujo D. R., Goulart H. A., Barcellos A. M., Cargnelutti R., Lenardão E. J., Perin G.. Oxone-Promoted Synthesis of 4-(Chalcogenyl)Isoquinoline-N-Oxides from Alkynylbenzaldoximes and Diorganyl Dichalcogenides. J. Org. Chem. 2021;86:1721–1729. doi: 10.1021/acs.joc.0c02525. - DOI - PubMed
- Anghinoni J. M., Ferreira S. S., Kazmierczak J. C., Perin G., Penteado F., Lenardão E. J.. Synthesis of Selenium-Decorated N-Oxide Isoquinolines: Arylseleninic Acids in Selenocyclization Reactions. J. Org. Chem. 2024;89:11272–11280. doi: 10.1021/acs.joc.4c00944. - DOI - PMC - PubMed
- Ding Q., Wu J.. Access to Functionalized Isoquinoline N-Oxides via Sequential Electrophilic Cyclization/Cross-Coupling Reactions. Adv. Synth. Catal. 2008;350:1850–1854. doi: 10.1002/adsc.200800301. - DOI
- Wang H., Zhu M., Ye S., Wu J.. Generation of Diverse Isoquinoline N-Oxides in an Aqueous System. RSC Adv. 2013;3:13626–13629. doi: 10.1039/c3ra42730g. - DOI
- Ding Q., Wang D., Sang X., Lin Y., Peng Y.. One-Pot Two-Step Synthesis of 1-Position Arylated 1,3-Disubstituted Isoquinoline N-Oxides. Tetrahedron. 2012;68:8869–8874. doi: 10.1016/j.tet.2012.08.039. - DOI
- Song J., Fan C., Liu G., Qiu G.. Generation of 1-Amino-Isoquinoline-N-Oxides via a Tandem Reaction of 2-Alkynylbenzaldoxime with Secondary Amines in the Presence of Silver(I) and Copper(I) Org. Chem. Front. 2014;1:1045–1049. doi: 10.1039/C4QO00209A. - DOI
- Li H., Liao L., Zhao X.. Organoselenium-Catalyzed Aza-Wacker Reactions: Efficient Access to Isoquinolinium Imides and an Isoquinoline N-Oxide. Synlett. 2019;30:1688–1692. doi: 10.1055/s-0039-1690103. - DOI
-
-
A report stated that the use of ketoxime substrates resulted in a decomposition of the starting material, see ref .
-
LinkOut - more resources
Full Text Sources
Miscellaneous

