SARM1 activation induces reversible mitochondrial dysfunction and can be prevented in human neurons by antisense oligonucleotides
- PMID: 40473123
- PMCID: PMC7617922
- DOI: 10.1016/j.nbd.2025.106986
SARM1 activation induces reversible mitochondrial dysfunction and can be prevented in human neurons by antisense oligonucleotides
Abstract
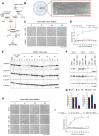
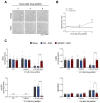
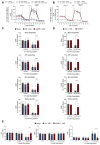
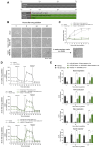
SARM1 is a key regulator of a conserved program of axon degeneration increasingly linked to human neurodegenerative diseases. Pathological SARM1 activation causes rapid NAD consumption, disrupting cellular homeostasis and leading to axon degeneration. In this study, we develop antisense oligonucleotides (ASOs) targeting human SARM1, demonstrating robust neuroprotection against morphological, metabolic, and mitochondrial impairment in human iPSC-derived dopamine neurons induced by the lethal neurotoxin vacor, a potent SARM1 activator. Furthermore, our findings reveal that axon fragmentation can be prevented, and mitochondrial dysfunction reversed using the NAD precursor nicotinamide, a form of vitamin B3, even after SARM1 activation has occurred, when neurons are already unhealthy. This research identifies ASOs as a promising therapeutic strategy to block SARM1, and provides an extensive characterisation and further mechanistic insights that demonstrate the reversibility of SARM1 toxicity in human neurons. It also identifies the SARM1 activator vacor as a specific and reversible neuroablative agent in human neurons.
Keywords: ASO; Axon degeneration; Mitochondrial dysfunction; Neuroablative; Nicotinamide; SARM1; Vacor.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest MPC consults for Nura Bio and Drishti Discoveries and the Coleman group is part funded by AstraZeneca for academic research projects but none of these activities relate to the study reported here. DLB has consulted for AstraZeneca and Lilly on behalf of Oxford University Innovation and received research grants from AstraZeneca and Lilly but none of these activities relate to these studies. HTZ is a full-time employee and stock holder of Ionis Pharmaceuticals, Inc. AL and PA-F are inventors on a patent application related to the subject matter of the publication. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Andrea Loreto reports financial support was provided by Wellcome Trust. David L Bennett reports financial support was provided by Wellcome Trust. Peter Arthur-Farraj reports financial support was provided by Wellcome Trust. Kaitlyn ML Cramb reports financial support was provided by Natural Sciences and Engineering Research Council of Canada. Richard Wade-Martins reports financial support was provided by Medical Research Council Dementias Platform UK. Andrea Loreto has patent pending to University of Cambridge. Peter Arthur-Farraj has patent pending to University of Cambridge. MPC consults for Nura Bio and Drishti Discoveries and the Coleman group is part funded by AstraZeneca for academic research projects but none of these activities relate to the study reported here. DLB has consulted for AstraZeneca and Lilly on behalf of Oxford University Innovation and received research grants from AstraZeneca and Lilly but none of these activities relate to these studies. HTZ is a full-time employee and stock holder of Ionis Pharmaceuticals, Inc. AL and PA-F are inventors on a patent application related to the subject matter of the publication. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Angeletti C, Amici A, Gilley J, Loreto A, Trapanotto AG, Antoniou C, Merlini E, Coleman MP, Orsomando G. SARM1 is a multi-functional NAD(P)ase with prominent base exchange activity, all regulated bymultiple physiologically relevant NAD metabolites. iScience. 2022;25:103812. doi: 10.1016/j.isci.2022.103812. - DOI - PMC - PubMed
-
- Beccano-Kelly DA, Cherubini M, Mousba Y, Cramb KML, Giussani S, Caiazza MC, Rai P, Vingill S, Bengoa-Vergniory N, Ng B, Corda G, et al. Calcium dysregulation combined with mitochondrial failure and electrophysiological maturity converge in Parkinson’s iPSC-dopamine neurons. iScience. 2023;26:107044. doi: 10.1016/j.isci.2023.107044. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

