Biosynthesis of fatty aldehydes and alcohols in the eye and their role in meibogenesis
- PMID: 40473210
- PMCID: PMC12269847
- DOI: 10.1016/j.jbc.2025.110330
Biosynthesis of fatty aldehydes and alcohols in the eye and their role in meibogenesis
Abstract
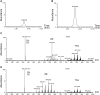
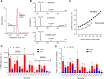
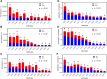
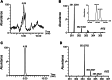
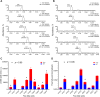
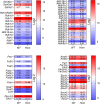
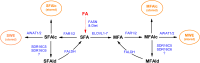
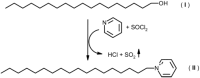
Fatty alcohols (FAlc) and aldehydes (FAld) are essential intermediates/precursors in the biosynthesis of lipids. However, elevated FAld levels were shown to be geno- and cytotoxic, thus requiring conversion into less toxic FAlc and fatty acids (FA). An increase in FAlc and FAld in tissues of patients with Sjögren-Larsson syndrome was reported before and repeatedly linked to inactivation of ALDH3A2, which oxidizes FAld in FA. Recently, we hypothesized that another group of enzymes, namely SDR16C5/SDR16C6 (EC 1.1.1.105), could control the balance between FA, FAlc, and FAld via a separate mechanism. In this study, we assessed the in vivo biosynthesis of FAlc and FAld in mammals using Meibomian glands (MG) of wild-type (WT) and Sdr16c5/Sdr16c6-null (Hom) mice as models. Lipids were extracted from MG of experimental animals and analyzed using LC/MS. Because of high reactivity and instability of FAld, the compounds were initially converted to stable, sodium borohydride-reduced 3-aminopyridine conjugates, while FAlc were analyzed as N-alkyl pyridinium ions. A wide range of saturated and unsaturated FAld, FAlc, and FA ranging from C3 to C28 and longer were found in MG of mice of both genotypes. Our experiments revealed a multifold upregulation of almost all detected straight chain, but not branched, FAlc in MG lipidomes of Hom mice, which implied a previously unknown ability of SDR16C5/SDR16C6 to oxidize a wide range of FAlc in FAld in vivo. We have concluded that SDR16C5/SDR16C6 plays a central, and selective, role in FA/FAlc/FAld metabolism in vivo and proposed a generalized mechanism of these reactions.
Keywords: Meibomian glands; chromatography/mass spectrometry; fatty acids; fatty alcohols; fatty aldehydes; lipids; lipogenesis; meibogenesis.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


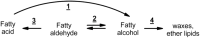
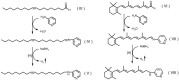
Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- Nicolaides N., Kaitaranta J.K., Rawdah T.N., Macy J.I., Boswell F.M., 3rd, Smith R.E. Meibomian gland studies: comparison of steer and human lipids. Invest. Ophthalmol. Vis. Sci. 1981;20:522–536. - PubMed
-
- Butovich I.A., Uchiyama E., McCulley J.P. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J. Lipid. Res. 2007;48:2220–2235. - PubMed
-
- Nicolaides N. The monoene and other Wax alcohols of human skin surface lipid and their relation to the fatty acids of this lipid. Lipids. 1967;2:266–275. - PubMed
-
- Nicolaides N., Fu H.C., Ansari M.N. Diester waxes in surface lipids of animal skin. Lipids. 1970;5:299–307. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

