Prenatal detection of congenital heart defects using the deep learning-based image and video analysis: protocol for Clinical Artificial Intelligence in Fetal Echocardiography (CAIFE), an international multicentre multidisciplinary study
- PMID: 40473283
- PMCID: PMC12142171
- DOI: 10.1136/bmjopen-2025-101263
Prenatal detection of congenital heart defects using the deep learning-based image and video analysis: protocol for Clinical Artificial Intelligence in Fetal Echocardiography (CAIFE), an international multicentre multidisciplinary study
Abstract
Introduction: Congenital heart defect (CHD) is a significant, rapidly emerging global problem in child health and a leading cause of neonatal and childhood death. Prenatal detection of CHDs with the help of ultrasound allows better perinatal management of such pregnancies, leading to reduced neonatal mortality, morbidity and developmental complications. However, there is a wide variation in reported fetal heart problem detection rates from 34% to 85%, with some low- and middle-income countries detecting as low as 9.3% of cases before birth. Research has shown that deep learning-based or more general artificial intelligence (AI) models can support the detection of fetal CHDs more rapidly than humans performing ultrasound scan. Progress in this AI-based research depends on the availability of large, well-curated and diverse data of ultrasound images and videos of normal and abnormal fetal hearts. Currently, CHD detection based on AI models is not accurate enough for practical clinical use, in part due to the lack of ultrasound data available for machine learning as CHDs are rare and heterogeneous, the retrospective nature of published studies, the lack of multicentre and multidisciplinary collaboration, and utilisation of mostly standard planes still images of the fetal heart for AI models. Our aim is to develop AI models that could support clinicians in detecting fetal CHDs in real time, particularly in nonspecialist or low-resource settings where fetal echocardiography expertise is not readily available.
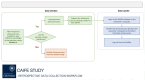
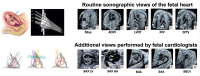
Methods and analysis: We have designed the Clinical Artificial Intelligence Fetal Echocardiography (CAIFE) study as an international multicentre multidisciplinary collaboration led by a clinical and an engineering team at the University of Oxford. This study involves five multicountry hospital sites for data collection (Oxford, UK (n=1), London, UK (n=3) and Southport, Australia (n=1)). We plan to curate 14 000 retrospective ultrasound scans of fetuses with normal hearts (n=13 000) and fetuses with CHDs (n=1000), as well as 2400 prospective ultrasound cardiac scans, including the proposed research-specific CAIFE 10 s video sweeps, from fetuses with normal hearts (n=2000) and fetuses diagnosed with major CHDs (n=400). This gives a total of 16 400 retrospective and prospective ultrasound scans from the participating hospital sites. We will build, train and validate computational models capable of differentiating between normal fetal hearts and those diagnosed with CHDs and recognise specific types of CHDs. Data will be analysed using statistical metrics, namely, sensitivity, specificity and accuracy, which include calculating positive and negative predictive values for each outcome, compared with manual assessment.
Ethics and dissemination: We will disseminate the findings through regional, national and international conferences and through peer-reviewed journals. The study was approved by the Health Research Authority, Care Research Wales and the Research Ethics Committee (Ref: 23/EM/0023; IRAS Project ID: 317510) on 8 March 2023. All collaborating hospitals have obtained the local trust research and development approvals.
Keywords: Artificial Intelligence; Congenital heart disease; Diagnostic Imaging; Echocardiography; Pregnant Women; Prenatal diagnosis.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures




References
-
- Zheleva B, Atwood JB. The invisible child: childhood heart disease in global health. Lancet. 2017;389:16–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
