Sacituzumab tirumotecan versus docetaxel for previously treated EGFR-mutated advanced non-small cell lung cancer: multicentre, open label, randomised controlled trial
- PMID: 40473437
- PMCID: PMC12139608
- DOI: 10.1136/bmj-2025-085680
Sacituzumab tirumotecan versus docetaxel for previously treated EGFR-mutated advanced non-small cell lung cancer: multicentre, open label, randomised controlled trial
Erratum in
-
Sacituzumab tirumotecan versus docetaxel for previously treated EGFR-mutated advanced non-small cell lung cancer: multicentre, open label, randomised controlled trial.BMJ. 2025 Nov 3;391:r2300. doi: 10.1136/bmj.r2300. BMJ. 2025. PMID: 41184034 No abstract available.
Abstract
Objective: To compare the efficacy and safety of sacituzumab tirumotecan (sac-TMT) with docetaxel in patients with locally advanced or metastatic epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) after previous treatment failure with EGFR-tyrosine kinase inhibitors and platinum based chemotherapy.
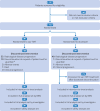
Design: Multicentre, open label, randomised controlled trial.
Setting: 48 centres in China, 1 September 2023 to 31 December 2024.
Participants: 137 adults (aged 18-75 years) with EGFR-mutated advanced or metastatic NSCLC after previous treatment failure with EGFR-tyrosine kinase inhibitors and platinum based chemotherapy.
Intervention: Patients were randomly assigned (2:1) to receive sac-TMT (5 mg/kg) on days 1 and 15 of each four week cycle, or docetaxel (75 mg/m2) on day 1 of each three week cycle. Patients in the docetaxel group were permitted to crossover to sac-TMT treatment on disease progression.
Main outcome measures: The primary endpoint was objective response rate as assessed by a blinded independent review committee (BIRC). The secondary endpoints included objective response rate assessed by the investigator; disease control rate, progression-free survival, time to response, and duration of response assessed by BIRC and the investigator; overall survival; and safety.
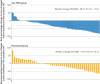
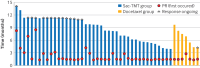
Results: 137 patients were randomised to receive sac-TMT (n=91) or docetaxel (n=46). Median follow-up was 12.2 months at the data cut-off for efficacy (31 December 2024). BIRC assessed objective response rate was significantly higher in the sac-TMT group (45% (41/91)) v docetaxel (16% (7/45)), with a difference of 29% (95% confidence interval (CI) 15% to 43%; one sided P<0.001). Median progression-free survival was longer with sac-TMT than with docetaxel assessed by BIRC (6.9 v 2.8 months; hazard ratio 0.30, 95% CI 0.20 to 0.46; one sided P<0.001) and the investigator (7.9 v 2.8 months; hazard ratio 0.23, 0.15 to 0.36; one sided P<0.001). The 12 month overall survival rate was 73% with sac-TMT and 54% with docetaxel (hazard ratio 0.49, 0.27 to 0.88; one sided P=0.007). After adjustment for crossover using the rank-preserving structural failure time model, sac-TMT also showed improved overall survival (hazard ratio 0.36, 0.20 to 0.66). Grade ≥3 treatment related adverse events were less frequent with sac-TMT than with docetaxel (56% v 72%), with no new safety signals identified.
Conclusions: Sac-TMT showed statistically significant and clinically meaningful improvements in objective response rate, progression-free survival, and overall survival compared with docetaxel, with a manageable safety profile in patients with EGFR-mutated locally advanced or metastatic NSCLC.
Trial registration: ClinicalTrials.gov NCT05631262.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from Sichuan Kelun-Biotech Biopharmaceutical, and partial support from the National Natural Science Foundation of China and Noncommunicable Chronic Diseases-National Science and Technology Major Project for the submitted work; JY, YD, XJ, and JG are employed by Sichuan Kelun-Biotech Biopharmaceutical YD, XJ, and JG hold company stock; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Kawaguchi T, Ando M, Asami K, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol 2014;32:1902-8. 10.1200/JCO.2013.52.4694. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
