Imaging Efficacy of [18F]CTT1057 PET/CT in Patients with Biochemically Recurrent Prostate Cancer: Results from GuidePath-A Phase 3, Prospective Multicenter Study
- PMID: 40473464
- PMCID: PMC12320562
- DOI: 10.2967/jnumed.124.269266
Imaging Efficacy of [18F]CTT1057 PET/CT in Patients with Biochemically Recurrent Prostate Cancer: Results from GuidePath-A Phase 3, Prospective Multicenter Study
Abstract
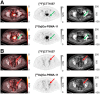
Improved diagnostic accuracy in patients with prostate cancer at first biochemical recurrence (BCR) with low prostate-specific antigen (PSA) levels is needed. This prospective study (GuidePath; NCT04838613) aimed to evaluate the imaging performance of the prostate-specific membrane antigen (PSMA)-targeted PET radiotracer [18F]CTT1057 to detect PSMA-positive lesions in patients diagnosed predominantly at first BCR. Methods: Eligible patients had a PSA of 0.2 ng/mL or greater after radical prostatectomy or an increase in PSA level of at least 2 ng/mL above nadir after radiation therapy. Patients received 370 MBq of [18F]CTT1057 and 150 MBq of [68Ga]Ga-PSMA-11 and underwent PET/CT 90 min (±30 min) and 50-100 after injection, respectively. [18F]CTT1057 images were assessed by 3 independent readers blinded to all clinical information. Coprimary endpoints were region-level correct localization rate (CLR) and patient-level positive predictive value (PPV) of [18F]CTT1057 to detect PSMA-positive lesions and were compared with a hierarchical composite truth standard (CTS). The CTS comprised 3 levels of standard-of-truth procedures (in order of priority): histopathology (CTS level 1); imaging, including at least 1 contrast-enhanced CT scan and 1 [68Ga]Ga-PSMA-11 PET/CT scan (CTS level 2); and a decrease in PSA level of 50% or greater 3 mo after radiation therapy (CTS level 3). For study success, the lower-bound 95% CI had to surpass 50% for region-level CLR and 20% for patient-level PPV for at least 2 of the 3 [18F]CTT1057 PET/CT readers. Results: Of 202 patients screened, 161 were evaluable for efficacy. Among these, 93.2% were experiencing their first BCR, 96.3% had received radical prostatectomy as initial definitive therapy, and baseline median PSA level was 0.4 ng/mL (interquartile range, 0.3-0.8 ng/mL). The imaging standard of truth was used for 159-160 patients (99%) across the 3 readers. Both coprimary endpoints were met. Region-level CLR ranged from 65.2% to 75.0% (lower-bound 95% CI, 53.4%-62.1%), and patient-level PPV ranged from 64.6% to 76.5% (lower-bound 95% CI, 51.8%-62.5%). Conclusion: [18F]CTT1057 met the predefined thresholds for region-level CLR and patient-level PPV in a clinically relevant patient cohort predominantly at first BCR with low PSA levels. [18F]CTT1057 is an accurate PSMA-targeted PET radiotracer for BCR detection.
Keywords: PET; [18F]CTT1057; biochemical recurrence; clinical trial; prostate-specific membrane antigen.
© 2025 by the Society of Nuclear Medicine and Molecular Imaging.
Figures



Similar articles
-
Imaging Efficacy of [18F]CTT1057 PET for the Detection of PSMA-Positive Tumors Using Histopathology as Standard of Truth: Results from the GuideView Phase 2/3 Prospective Multicenter Study.J Nucl Med. 2025 Aug 1;66(8):1232-1238. doi: 10.2967/jnumed.124.269007. J Nucl Med. 2025. PMID: 40473463 Free PMC article. Clinical Trial.
-
The Role of Prostate-specific Membrane Antigen Positron Emission Tomography for Assessment of Local Recurrence and Distant Metastases in Patients with Biochemical Recurrence of Prostate Cancer After Definitive Treatment: A Systematic Review and Meta-analysis.Eur Urol. 2025 Aug;88(2):129-141. doi: 10.1016/j.eururo.2025.05.006. Epub 2025 May 19. Eur Urol. 2025. PMID: 40393864 Review.
-
PSMA-PET/CT Findings in Patients With High-Risk Biochemically Recurrent Prostate Cancer With No Metastatic Disease by Conventional Imaging.JAMA Netw Open. 2025 Jan 2;8(1):e2452971. doi: 10.1001/jamanetworkopen.2024.52971. JAMA Netw Open. 2025. PMID: 39752157 Free PMC article.
-
Multicenter External Validation and Optimization of a Proposed Nomogram for Prostate-Specific Membrane Antigen PET/CT Accuracy in Biochemical Recurrence.Prostate. 2025 Aug;85(11):1016-1023. doi: 10.1002/pros.24910. Epub 2025 May 6. Prostate. 2025. PMID: 40329523
-
The role of 68Ga-PSMA PET/CT scan in biochemical recurrence after primary treatment for prostate cancer: a systematic review of the literature.Minerva Urol Nefrol. 2018 Oct;70(5):462-478. doi: 10.23736/S0393-2249.18.03081-3. Epub 2018 Apr 17. Minerva Urol Nefrol. 2018. PMID: 29664244
References
-
- Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. - PubMed
-
- Isbarn H, Wanner M, Salomon G, et al. Long-term data on the survival of patients with prostate cancer treated with radical prostatectomy in the prostate-specific antigen era. BJU Int. 2010;106:37–43. - PubMed
-
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. - PubMed
-
- Bott SRJ. Management of recurrent disease after radical prostatectomy. Prostate Cancer Prostatic Dis. 2004;7:211–216. - PubMed
-
- Beresford MJ, Gillatt D, Benson RJ, Ajithkumar T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin Oncol (R Coll Radiol). 2010;22:46–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
