CPK28-mediated Ca2+ signaling regulates STOP1 localization and accumulation to facilitate plant aluminum resistance
- PMID: 40473612
- PMCID: PMC12141487
- DOI: 10.1038/s41467-025-60427-8
CPK28-mediated Ca2+ signaling regulates STOP1 localization and accumulation to facilitate plant aluminum resistance
Abstract
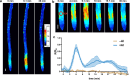
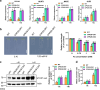
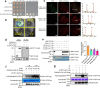
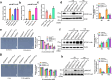
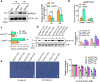
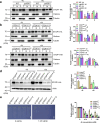
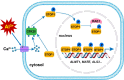
The transcription factor SENSITIVE TO PROTON RHIZOTOXICITY 1 (STOP1) functions as a crucial integrator of plant responses to various stresses, including aluminum (Al) stress. Its stability and accumulation are modulated by stress-specific post-translational mechanisms such as phosphorylation and ubiquitination. However, the upstream signaling mechanisms governing these modifications remain poorly understood. Here, we reveal that Ca2+ signaling and Ca2+-dependent phosphorylation are essential for Al stress-responsive regulation of STOP1. Al exposure specifically induces rapid, spatio-temporally defined biphasic Ca2+ signals in Arabidopsis roots and concomitantly activates the Ca2+-dependent kinase CPK28. Al-activated CPK28 phosphorylates STOP1 at Ser163, a modification that promotes the nuclear localization of STOP1 and prevents its degradation by inhibiting its interaction with the F-box protein RAE1. This phosphorylation enhances STOP1 accumulation and Al resistance. Our findings identify Ser163 phosphorylation as a key molecular switch and establish a Ca2+-CPK28-STOP1 signaling axis critical for plant adaptation to Al stress.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Ca2+-dependent cytoplasmic and nuclear phosphorylation of STOP1 by CPK21 and CPK23 confers ALMT1-dependent aluminum resistance.Nat Commun. 2025 Jun 5;16(1):5225. doi: 10.1038/s41467-025-60426-9. Nat Commun. 2025. PMID: 40473618 Free PMC article.
-
Degradation of STOP1 mediated by the F-box proteins RAH1 and RAE1 balances aluminum resistance and plant growth in Arabidopsis thaliana.Plant J. 2021 Apr;106(2):493-506. doi: 10.1111/tpj.15181. Epub 2021 Mar 10. Plant J. 2021. PMID: 33528836
-
H2O2 negatively regulates aluminum resistance via oxidation and degradation of the transcription factor STOP1.Plant Cell. 2024 Feb 26;36(3):688-708. doi: 10.1093/plcell/koad281. Plant Cell. 2024. PMID: 37936326 Free PMC article.
-
Aluminum resistance in plants: A critical review focusing on STOP1.Plant Commun. 2025 Feb 10;6(2):101200. doi: 10.1016/j.xplc.2024.101200. Epub 2024 Dec 2. Plant Commun. 2025. PMID: 39628052 Free PMC article. Review.
-
Synergistic and antagonistic pleiotropy of STOP1 in stress tolerance.Trends Plant Sci. 2021 Oct;26(10):1014-1022. doi: 10.1016/j.tplants.2021.06.011. Epub 2021 Jul 9. Trends Plant Sci. 2021. PMID: 34253485 Review.
References
-
- Sadhukhan, A., Kobayashi, Y., Iuchi, S. & Koyama, H. Synergistic and antagonistic pleiotropy of STOP1 in stress tolerance. Trends Plant Sci.26, 1014–1022 (2021). - PubMed
-
- Sadhukhan, A. et al. Sensitive to proton rhizotoxicity1 regulates salt and drought tolerance of Arabidopsis thaliana through transcriptional regulation of CIPK23. Plant Cell Physiol.60, 2113–2126 (2019). - PubMed
-
- Tian, W. H. et al. A transcription factor STOP1-centered pathway coordinates ammonium and phosphate acquisition in Arabidopsis. Mol. Plant14, 1554–1568 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

