An archaeal nucleoid-associated protein binds an essential motif in DNA replication origins
- PMID: 40473630
- PMCID: PMC12141716
- DOI: 10.1038/s41467-025-60618-3
An archaeal nucleoid-associated protein binds an essential motif in DNA replication origins
Abstract
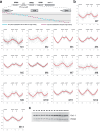
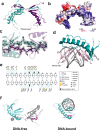
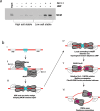
DNA replication typically has defined start sites, or replication origins, which are designated by their recognition by specific initiator proteins. In addition to initiators, general chromatin or nucleoid-associated proteins have been shown to play roles in modulating origin efficiency in eukaryotes and bacteria. The role of chromatin proteins in origin function in the archaeal domain of life is poorly understood. Here, we describe a dissection of sequences elements required for in vivo function of an archaeal DNA replication origin. Our data reveal a hitherto uncharacterized sequence element, the ucm, is required for origin activity. We identify a protein, UBP, that interacts with the ucm and additionally with hundreds of other sites on the genome. We solve the crystal structure of UBP alone and in complex with ucm DNA, and further show that UBP interacts with the MCM replicative helicase. Taken together, our data provide evidence that UBP functions as a general nucleoid-associated protein that plays a key role in facilitating the egress of the MCM replicative helicase from DNA replication origins.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
The rad50 signature motif: essential to ATP binding and biological function.J Mol Biol. 2004 Jan 23;335(4):937-51. doi: 10.1016/j.jmb.2003.11.026. J Mol Biol. 2004. PMID: 14698290
-
Cell cycle-dependent TICRR/TRESLIN and MTBP chromatin binding mechanisms and patterns.Genome Biol. 2025 Jul 7;26(1):194. doi: 10.1186/s13059-025-03665-9. Genome Biol. 2025. PMID: 40624716 Free PMC article.
-
Analysis of cis and trans Requirements for DNA Replication at the Right-End Hairpin of the Human Bocavirus 1 Genome.J Virol. 2016 Aug 12;90(17):7761-77. doi: 10.1128/JVI.00708-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334591 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Selenium for preventing cancer.Cochrane Database Syst Rev. 2018 Jan 29;1(1):CD005195. doi: 10.1002/14651858.CD005195.pub4. Cochrane Database Syst Rev. 2018. PMID: 29376219 Free PMC article.
References
-
- Jacob, F., Brenner, S. & Cuzin, F. On the regulation of DNA replication in bacteria. Cold Spring Harb. Symp. Quant. Biol.28, 329–348 (1963).
-
- Bell, S. P. & Dutta, A. DNA replication in eukaryotic cells. Annu. Rev. Biochem71, 333–374 (2002). - PubMed
MeSH terms
Substances
Grants and funding
- 086045/Z/08/Z/Wellcome Trust (Wellcome)
- R01GM135178/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- R01 GM135178/GM/NIGMS NIH HHS/United States
- R35GM152171/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- R01 GM125579/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

