Bacterial membrane nanovesicles encapsulating prodrug assemblies combine chemical and immunological therapies for chronic bacterial infection
- PMID: 40473640
- PMCID: PMC12141615
- DOI: 10.1038/s41467-025-60570-2
Bacterial membrane nanovesicles encapsulating prodrug assemblies combine chemical and immunological therapies for chronic bacterial infection
Abstract
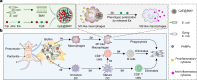
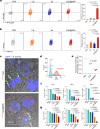
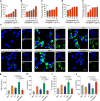
Overcoming challenges in drug targeting and modulating the immunosuppressive microenvironment are critical for treating chronic bacterial infections, which are often characterized by intracellular bacteria and biofilms. To overcome these barriers, we report a multifunctional nanomedicine (CpE@BMV). The prodrug conjugate (CpE), composed of two phenylboronic acid-modified ciprofloxacin (Cip-pba) molecules and ellagic acid (Ea), self-assembles due to its hydrophobic nature and π-π stacking. Bacterial membrane vesicles (BMVs) derived from Escherichia coli aid in CpE assembly and structural stabilization. Upon administration, pathogen-associated molecular patterns on CpE@BMV engage toll-like receptors on macrophages, activating these cells and enhancing their phagocytic response. Once internalized, CpE responds to elevated intracellular H₂O₂ levels, releasing Cip to eliminate intracellular bacteria. Additionally, Ea scavenges excess reactive oxygen species in inflamed macrophages and modulates the expression of inflammatory factors, preventing an exaggerated inflammatory response. The CpE@BMV formulation also penetrates biofilms, eliminating bacteria and releasing antigens. These antigens are transported to draining lymph nodes, where they induce dendritic cell maturation and trigger a robust T and B cell-mediated immune response, helping restore immune balance and combat pathogens effectively in female mouse models. Therefore, our CpE@BMV provide an efficient strategy combining chemical and immunological therapies for chronic bacterial infection management.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




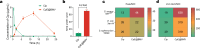
References
MeSH terms
Substances
Grants and funding
- 52422307/National Natural Science Foundation of China (National Science Foundation of China)
- 22275043/National Natural Science Foundation of China (National Science Foundation of China)
- 52203184/National Natural Science Foundation of China (National Science Foundation of China)
- 52293383/National Natural Science Foundation of China (National Science Foundation of China)
- LR24H180001/Natural Science Foundation of Zhejiang Province (Zhejiang Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

