Chimeric hemagglutinin and M2 mRNA vaccine for broad influenza subtype protection
- PMID: 40473642
- PMCID: PMC12141470
- DOI: 10.1038/s41541-025-01178-x
Chimeric hemagglutinin and M2 mRNA vaccine for broad influenza subtype protection
Abstract
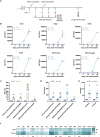
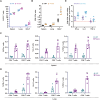
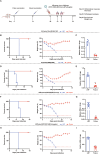
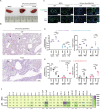
Since multiple and unpredicted influenza viruses cause seasonal epidemics and even high-risk pandemics, developing a universal influenza vaccine is essential to provide broad protection against various influenza subtypes. Combined with the mRNA lipid nanoparticle-encapsulated (mRNA-LNP) vaccine platform and chimeric immunogen strategy, we developed a novel cocktail mRNA vaccine encoding chimeric HAs (cH5/1-BV, cH7/3) and intact M2 (termed Fluaxe), which confers broad protection against major circulating IAVs and IBVs, as well as highly pathogenic avian influenza. Two-dose intramuscular immunization of Fluaxe in mice elicited cross-reactive neutralizing antibodies, T cell responses, and long-lived immunity, resulting in robust protection against multiple lethal influenza virus infections and severe acute lung injuries. In particular, intramuscular administration stimulated systemic immunity together with a prominent lung tropism of memory cells. Moreover, Fluaxe immunization inhibited the inflammatory response induced by influenza infection. In summary, we conclude that Fluaxe can elicit broad cross-protection against numerous influenza subtypes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.C., Y.D. and D.Y. are co-inventors on pending patent applications related to the IAV mRNA vaccine. N.L., Q.Z., Y.D., C.H., L.H., C.C., Y.W., H.C. and W.Z. are employees of RinuaGene Biotechnology Co., Ltd. All other authors declare no competing interests.
Figures


Similar articles
-
Recombinant parainfluenza virus 5 expressing clade 2.3.4.4b H5 hemagglutinin protein confers broad protection against H5Ny influenza viruses.J Virol. 2024 Mar 19;98(3):e0112923. doi: 10.1128/jvi.01129-23. Epub 2024 Feb 2. J Virol. 2024. PMID: 38305155 Free PMC article.
-
Vaccine Efficacy of Inactivated, Chimeric Hemagglutinin H9/H5N2 Avian Influenza Virus and Its Suitability for the Marker Vaccine Strategy.J Virol. 2017 Feb 28;91(6):e01693-16. doi: 10.1128/JVI.01693-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077631 Free PMC article.
-
Influenza 5xM2e mRNA lipid nanoparticle vaccine confers broad immunity and significantly enhances the efficacy of inactivated split vaccination when coadministered.J Immunol. 2025 Jan 1;214(1):104-114. doi: 10.1093/jimmun/vkae013. J Immunol. 2025. PMID: 40073270
-
Development of an mRNA vaccine against a panel of heterologous H1N1 seasonal influenza viruses using a consensus hemagglutinin sequence.Emerg Microbes Infect. 2023 Dec;12(1):2202278. doi: 10.1080/22221751.2023.2202278. Emerg Microbes Infect. 2023. PMID: 37067355 Free PMC article.
-
Lipid nanoparticle-encapsulated DNA vaccine confers protection against swine and human-origin H1N1 influenza viruses.mSphere. 2024 Aug 28;9(8):e0028324. doi: 10.1128/msphere.00283-24. Epub 2024 Aug 1. mSphere. 2024. PMID: 39087764 Free PMC article.
References
-
- Luksza, M. & Lässig, M. A predictive fitness model for influenza. Nature507, 57–61 (2014). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

