Chimeric hemagglutinin and M2 mRNA vaccine for broad influenza subtype protection
- PMID: 40473642
- PMCID: PMC12141470
- DOI: 10.1038/s41541-025-01178-x
Chimeric hemagglutinin and M2 mRNA vaccine for broad influenza subtype protection
Abstract
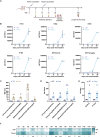
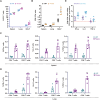
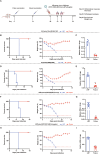
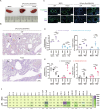
Since multiple and unpredicted influenza viruses cause seasonal epidemics and even high-risk pandemics, developing a universal influenza vaccine is essential to provide broad protection against various influenza subtypes. Combined with the mRNA lipid nanoparticle-encapsulated (mRNA-LNP) vaccine platform and chimeric immunogen strategy, we developed a novel cocktail mRNA vaccine encoding chimeric HAs (cH5/1-BV, cH7/3) and intact M2 (termed Fluaxe), which confers broad protection against major circulating IAVs and IBVs, as well as highly pathogenic avian influenza. Two-dose intramuscular immunization of Fluaxe in mice elicited cross-reactive neutralizing antibodies, T cell responses, and long-lived immunity, resulting in robust protection against multiple lethal influenza virus infections and severe acute lung injuries. In particular, intramuscular administration stimulated systemic immunity together with a prominent lung tropism of memory cells. Moreover, Fluaxe immunization inhibited the inflammatory response induced by influenza infection. In summary, we conclude that Fluaxe can elicit broad cross-protection against numerous influenza subtypes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.C., Y.D. and D.Y. are co-inventors on pending patent applications related to the IAV mRNA vaccine. N.L., Q.Z., Y.D., C.H., L.H., C.C., Y.W., H.C. and W.Z. are employees of RinuaGene Biotechnology Co., Ltd. All other authors declare no competing interests.
Figures


References
-
- Luksza, M. & Lässig, M. A predictive fitness model for influenza. Nature507, 57–61 (2014). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

