Loss of state transitions in Bryopsidales macroalgae and kleptoplastic sea slugs (Gastropoda, Sacoglossa)
- PMID: 40473784
- PMCID: PMC12141491
- DOI: 10.1038/s42003-025-08305-3
Loss of state transitions in Bryopsidales macroalgae and kleptoplastic sea slugs (Gastropoda, Sacoglossa)
Abstract
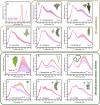
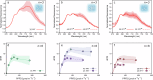
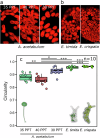
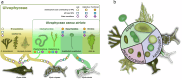
Green macroalgae within the order Bryopsidales lack the fundamental photoprotective mechanisms of green algae, the xanthophyll cycle and energy-dependent dissipation of excess light. Here, by measuring chlorophyll fluorescence at 77 K after specific light treatments, we show that Bryopsidales algae also lack state transitions, another ubiquitous photoprotection mechanism present in other green algae. Certain Sacoglossa sea slugs can feed on Ulvophyceae algae, including some Bryopsidales, and steal chloroplasts - kleptoplasts - that remain functional inside the animal cells for months without the support of the algal nucleus. Our data reveal that the state transition capacity is not retained in the kleptoplasts of the sea slugs, and we provide evidence that the loss is caused by structural changes during their incorporation by the animals. Enforced chloroplast sphericity was observed in all studied kleptoplastic associations, and we propose that it is a fundamental property supporting long-term retention of kleptoplasts in photosynthetic sea slugs.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: This study was performed in accordance with EU legislation and directives concerning scientific research on animals, including the 3 R principles. Ethical approval is not required for studies conducted with non-cephalopod invertebrates. Wild algae and sea slugs were collected from non-protected marine coastal areas. Laboratory-reared specimens used in this work were maintained in optimized life-support systems to ensure animal welfare.
Figures




References
-
- de Negri, A. & de Negri, G. Farbstoff aus Elysia viridis. Ber. Dtsch. Chem. Ges.9, 84 (1876).
MeSH terms
LinkOut - more resources
Full Text Sources

