TRIM65 regulates glucose metabolic reprogramming to promote glioma cell proliferation via ubiquitination and degradation of AMPK
- PMID: 40473847
- PMCID: PMC12141518
- DOI: 10.1038/s41698-025-00964-z
TRIM65 regulates glucose metabolic reprogramming to promote glioma cell proliferation via ubiquitination and degradation of AMPK
Abstract
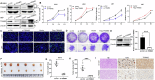
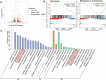
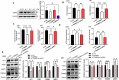
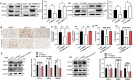
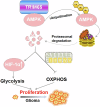
Glioma is the most common primary malignant brain tumor with high mortality and poor prognosis. Aerobic glycolysis is crucial for the malignant behavior of glioma by promoting their growth. Tripartite motif containing 65 (TRIM65) as an E3 ubiquitin ligase has been implicated in tumor progression, but its role and regulatory mechanism on aerobic glycolysis in glioma remains unclear. Here, it was demonstrated that TRIM65 was highly expressed in human glioma tissues and associated with poor prognosis. Moreover, TRIM65 knockdown inhibited the glioma cells proliferation in vitro and in vivo. RNA sequencing and biological verifications were performed to elucidate a novel mechanism underlying TRIM65 silencing attenuated glycolysis and enhanced OXPHOX to suppress the growth of glioma cells. Subsequently, we found that TRIM65 interacted with AMPK, a metabolic sensor, and mediated its K48-linkage ubiquitination and degradation though proteasomal pathway, thereby regulating HIF-1α-induced glycolysis. Importantly, the inhibitory effect of TRIM65 silencing on glycolysis was abrogated by AMPK knockdown or HIF-1α overexpression, indicating glucose metabolic reprogramming by TRIM65 is dependent on AMPK and HIF-1α pathway. These results reveal a new role for TRIM65/AMPK/HIF-1α axis in glioma cell proliferation and aerobic glycolysis, suggesting that TRIM65 may be a potential therapeutic target for intervention of glioma.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
Grants and funding
- 82303229/National Natural Science Foundation of China
- 82260173/National Natural Science Foundation of China
- 81472371/National Natural Science Foundation of China
- 82260626/National Natural Science Foundation of China
- 20232BAB216078/Jiangxi Provincial Natural Science Foundation
- 20212BDH81020/Jiangxi Provincial Natural Science Foundation
- 20224ACB206014/Jiangxi Provincial Natural Science Foundation
- 20224BAB216052/Jiangxi Provincial Natural Science Foundation
- 2023ZD001/Jiangxi Provincial Health Commission Science and Technology Plan Project
- 202310128/Jiangxi Provincial Health Commission Science and Technology Plan Project
- 2022A346/Jiangxi Provincial Traditional Chinese Medicine Science and Technology Plan Project
LinkOut - more resources
Full Text Sources

