Enhancing Bone Scaffold Fabrication: A Comparative Study of Manual Casting and Automated 3D Bioprinting
- PMID: 40473909
- PMCID: PMC12391189
- DOI: 10.1007/s10439-025-03752-9
Enhancing Bone Scaffold Fabrication: A Comparative Study of Manual Casting and Automated 3D Bioprinting
Abstract
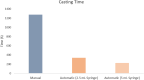
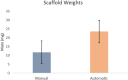
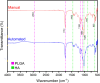
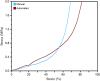
While fabrication of bone scaffolds is important for the development of tissue engineering, traditional techniques have typically been prone to either scaling or reproducibility issues. This paper highlights a strategy for automated 3D printing and bioprinting techniques that enhance precision and efficiency in the production of PLGA-HA scaffolds. We realized significant improvements in efficiency, reproducibility, and scalability through optimization of 3D printing parameters, improvement of material handling, and refinement of the fabrication process. Precise measurement consequently minimized material waste; the introduction of a mesh filter allowed for high-throughput experimentation without compromising the integrity of individual scaffolds, streamlining the workflow. Combining automated casting with state-of-the-art 3D bioprinting, our experimental methodology precisely applied the bioactive materials, reducing the processing time fivefold and enhancing precision. Besides, automated casting produced thicker, better-quality scaffolds averaging 0.02354 g, which is against 0.01169 g using the manual approach, effectively doubling the retention of the PLGA-HA coating on a PVA mold. Excellent cell viability and adhesion on automated scaffolds have been further underlined for application in tissue engineering during in vitro studies using multipotent mesenchymal stromal cells. Although conventional techniques, such as injection molding, are standard for large lots, 3D printing has advantages in scaffold fabrication regarding control over geometry and homogeneous material properties. Equally important, these characteristics are necessary to achieve repeatable and up-scaled experimental results.
Keywords: 3D printing; Automation; Bioprinting; Bone regeneration; In vitro; Tissue engineering.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have no competing financial or non-financial interests to declare that are relevant to the content of this article.
Figures




References
-
- Kannus, P., J. Parkkari, H. Sievänen, A. Heinonen, I. Vuori, and M. Järvinen. Epidemiology of hip fractures. Bone. 18(1 Suppl):57S-63S, 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

