tRF16 affects NFKBIA stability and promotes osteoarthritis progression by regulating ALKBH5 expression in m6A-dependent manner
- PMID: 40473927
- PMCID: PMC12141618
- DOI: 10.1038/s42003-025-08299-y
tRF16 affects NFKBIA stability and promotes osteoarthritis progression by regulating ALKBH5 expression in m6A-dependent manner
Abstract
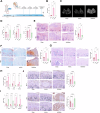
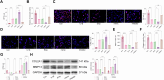
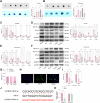
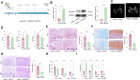
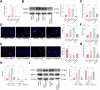
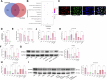
This study aimed to investigate explore the role of tRF16-ALKBH5 in osteoarthritis (OA). Differential expression of tRF in normal and OA tissues was analyzed using sequencing. OA rats model was established by destabilization of the medial meniscus and anterior cruciate ligament transaction surgeries. The tRF-16 inhibitor or mimic was injected into OA rats and OA symptoms were analyzed. We analyzed the m6A levels in tRF16 inhibitor-treated rat cartilage tissues, ALKBH5 levels, and the binding relationship between tRF16 and ALKBH5. The effect of ALKBH5 on OA rats was analyzed using specific antagonists. Chondrocytes were extracted to establish an OA cell model using IL-1β induction. The effects of tRF16, ALKBH5, and downstream genes on chondrocyte viability were verified. tRF-16 was overexpressed in OA patients and rat models. tRF16 inhibitor improved the symptoms of OA rats and inhibited autophagy and extracellular matrix degradation in IL-1β-induced chondrocytes. tRF16 reduced ALKBH5 expression by targeting ALKBH5, decreased NFKBIA mRNA stability, and activated the NF-kB pathway, thus exacerbating OA progression. Collectively, by binding to ALKBH5, tRF16 promotes the degradation of ALKBH5 and impairs the maintenance of NFKBIA mRNA stability by ALKBH5, promotes the nuclear translocation of phos-p65, leads to the secretion of inflammatory factors, exacerbates OA symptoms.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The studies involving human participants were reviewed and approved by the Ethics Committee and Institutional Review Board of the Third Affiliated Hospital of Nanjing Medical University ([2021]KY203-01). Written informed consent for participation in this study was provided by the participants. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Third Affiliated Hospital of Nanjing Medical University.
Figures

Similar articles
-
Inflammatory microenvironment promotes extracellular matrix degradation of chondrocytes through ALKBH5-dependent Runx2 m6A modification in the pathogenesis of osteoarthritis.Int Immunopharmacol. 2025 Jan 10;144:113638. doi: 10.1016/j.intimp.2024.113638. Epub 2024 Nov 23. Int Immunopharmacol. 2025. PMID: 39580858
-
TRIM8 enhances chondrocyte ferroptosis by inhibiting YTHDF2-m6A mediated SREBF2 mRNA degradation to promote OA progression.Int Immunopharmacol. 2025 Apr 16;152:114441. doi: 10.1016/j.intimp.2025.114441. Epub 2025 Mar 11. Int Immunopharmacol. 2025. PMID: 40073810
-
IL-1β receptor antagonist (IL-1Ra) combined with autophagy inducer (TAT-Beclin1) is an effective alternative for attenuating extracellular matrix degradation in rat and human osteoarthritis chondrocytes.Arthritis Res Ther. 2019 Jul 10;21(1):171. doi: 10.1186/s13075-019-1952-5. Arthritis Res Ther. 2019. PMID: 31291980 Free PMC article.
-
Danshensu inhibits the IL-1β-induced inflammatory response in chondrocytes and osteoarthritis possibly via suppressing NF-κB signaling pathway.Mol Med. 2021 Jul 20;27(1):80. doi: 10.1186/s10020-021-00329-9. Mol Med. 2021. PMID: 34284715 Free PMC article.
-
Myrislignan ameliorates the progression of osteoarthritis: An in vitro and in vivo study.Int Immunopharmacol. 2024 Oct 25;140:112887. doi: 10.1016/j.intimp.2024.112887. Epub 2024 Aug 7. Int Immunopharmacol. 2024. PMID: 39116493
References
-
- Martel-Pelletier, J. et al. Osteoarthritis. Nat. Rev. Dis. Prim.2, 16072 (2016). - PubMed
-
- Karsdal, M. A. et al. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthr. Cartil.24, 2013–2021 (2016). - PubMed
-
- Glyn-Jones, S. et al. Osteoarthritis. Lancet386, 376–387 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

