Infant Parent Support (IPS): a multidisciplinary intervention to improve the mental health of children with a social worker - a study protocol for a feasibility randomised controlled trial with embedded process evaluation
- PMID: 40474316
- PMCID: PMC12139322
- DOI: 10.1186/s40814-025-01616-6
Infant Parent Support (IPS): a multidisciplinary intervention to improve the mental health of children with a social worker - a study protocol for a feasibility randomised controlled trial with embedded process evaluation
Abstract
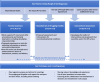
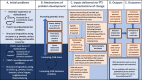
Background: In many families where children have a social worker, parents have experienced challenges in their own childhoods or have neurodevelopmental conditions. These families often endure significant stress, which is frequently worsened by financial or housing challenges. This added pressure can strain relationships and increase the risk of child maltreatment, as well as contribute to mental health issues in children. Relationship-focused interventions show promise in preventing child maltreatment, although there are currently no interventions that simultaneously address neurodevelopmental conditions and the impact of poverty. We have co-produced, alongside parent experts-by-experience, local stakeholders, and infant mental health practitioners, a new service called Infant Parent Support (IPS). IPS will i) adopt a relationship-focused approach to comprehensive understanding of family functioning, ii) incorporate child and parent mental health and neurodevelopmental awareness, and iii) ensure a poverty aware approach throughout. The aim of this phase is to investigate the feasibility of a definitive Randomised Controlled Trial (RCT) of IPS compared with services-as-usual (SAU).
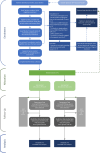
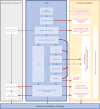
Methods: The study settings are social care services in two local authorities: Glasgow City Council (Scotland) and the London Borough of Bromley (England). Our target population is children on a 'child in need' plan (or the Scottish equivalent) and eligible participants are families where i) the infant(s) are aged 0-5 years and ii) the family has an allocated social worker plus a multi-agency support plan. Thirty participants will be identified by social workers and randomised to receive either IPS or SAU. Families randomised to IPS will receive an intensive multidisciplinary attachment-focused assessment that provides a foundation for relationship-focused interventions. IPS will incorporate child and parent mental health and neurodevelopmental awareness and ensure a poverty aware approach throughout. Families randomised to SAU will receive the assessment and support that social care services normally implement. We will utilise a pre-post and 3/6-month follow-up design with embedded mixed-method process evaluation and exploratory economic analysis. The primary objective is to assess if enough families can be recruited, randomised, and retained in the trial such that a full-scale RCT is likely to be feasible. The secondary objectives are to assess the acceptability and feasibility of the planned outcome measures and the IPS intervention to families and professionals.
Conclusions: A service like IPS, that uses a relationship-focused approach to child and parent mental health, neurodevelopmental and money/housing problems, has never previously been tested. Therefore, there are several areas of uncertainty that need to be addressed before moving onto a definitive RCT. TRIAL REGISTRATION {2A AND 2B}: Registered in ClinicalTrials.gov Identifier: NCT06003582. Co-production and Feasibility RCT of Intervention to Improve the Mental Health of Children with a Social Worker. Registered 22/08/2023. https://classic.
Clinicaltrials: gov/ct2/show/NCT06003582 .
Keywords: Children in need; Co-production; Feasibility randomised controlled trial (fRCT); Health economics; Infant mental health; Lived experience; Resilience; Social care; Vulnerable families.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval and Consent to Participate {24}: Ethical approval has been granted by the College of Medical, Veterinary and Life Sciences Ethics Committee for Non-Clinical Research Involving Human Subjects, University of Glasgow (Ref. 200220080). Informed written consent will be obtained from all participants. Participants may withdraw from the study at any point without giving a reason; however, we will record and monitor any reasons that are given. Participants will be consented to the trial by research staff prior to all data collection and we will seek permission to link to their routine data. Model consent forms are available on request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. Consent for Publication {32}: Not applicable. Competing Interests {28}: MF is Partnerships and Development Director at NSPCC. NSPCC delivers the intervention arm of the trial. MF has been involved in the UK development of the interventions.
Figures
Similar articles
-
An intervention for parents with severe personality difficulties whose children have mental health problems: a feasibility RCT.Health Technol Assess. 2020 Mar;24(14):1-188. doi: 10.3310/hta24140. Health Technol Assess. 2020. PMID: 32174297 Free PMC article. Clinical Trial.
-
An intervention to improve the quality of life in children of parents with serious mental illness: the Young SMILES feasibility RCT.Health Technol Assess. 2020 Nov;24(59):1-136. doi: 10.3310/hta24590. Health Technol Assess. 2020. PMID: 33196410 Free PMC article. Clinical Trial.
-
A modified video-feedback intervention for carers of foster children aged 6 years and under with reactive attachment disorder: a feasibility study and pilot RCT.Health Technol Assess. 2022 Aug;26(35):1-106. doi: 10.3310/SLIZ1119. Health Technol Assess. 2022. PMID: 35959710 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Behaviour change interventions to reduce risky substance use and improve mental health in children in care: the SOLID three-arm feasibility RCT.Southampton (UK): NIHR Journals Library; 2020 Sep. Southampton (UK): NIHR Journals Library; 2020 Sep. PMID: 32960519 Free Books & Documents. Review.
References
-
- Department for Education. Help, protection, education: concluding the children in need review. 2019. Available at https://assets.publishing.service.gov.uk/media/5d03c221e5274a0b725bcff3/.... Accessed 15 Dec 2023.
-
- Skinner G, Bywaters P, Bilson A, Duschinsky R, Clements K, Hutchinson D. The ‘toxic trio’ (domestic violence, substance misuse and mental ill-health): How good is the evidence base? Child Youth Serv Rev. 2021;120:105678. 10.1016/j.childyouth.2020.105678.
-
- Broadhurst K, Mason C. Birth parents and the collateral consequences of court-ordered child removal: towards a comprehensive framework. International Journal of Law, Policy and the Family. 2017;31(1):41–59. 10.1093/lawfam/ebw013.
Associated data
LinkOut - more resources
Full Text Sources
Medical