Equivalence of SYN008 versus omalizumab in patients with refractory chronic spontaneous urticaria: A multicenter, randomized, double-blind, parallel-group, active-controlled phase III study
- PMID: 40474341
- PMCID: PMC12369717
- DOI: 10.1097/CM9.0000000000003593
Equivalence of SYN008 versus omalizumab in patients with refractory chronic spontaneous urticaria: A multicenter, randomized, double-blind, parallel-group, active-controlled phase III study
Conflict of interest statement
This study was supported by China Shijiazhuang Pharmaceutical Group Co., Ltd., China.
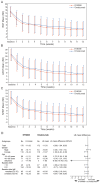
Figures
References
-
- Kolkhir P, Giménez-Arnau AM, Kulthanan K, Peter J, Metz M, Maurer M. Urticaria. Nat Rev Dis Primers 2022;8:61. doi: 10.1038/s41572-022-00389-z. - PubMed
-
- Zuberbier T Abdul Latiff AH Abuzakouk M Aquilina S Asero R Baker D, et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022;77:734–766. doi: 10.1111/all.15090. - PubMed
-
- FDA . XOLAIR® (omalizumab) label. 2022. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/103976s5238lbl.... [Last accessed on 24 June, 2024].
-
- Zhao ZT Ji CM Yu WJ Meng L Hawro T Wei JF, et al. Omalizumab for the treatment of chronic spontaneous urticaria: A meta-analysis of randomized clinical trials. J Allergy Clin Immunol 2016;137:1742–1750.e1744. doi: 10.1016/j.jaci.2015.12.1342. - PubMed
LinkOut - more resources
Full Text Sources

