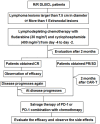
An exploration of the initiation time and patient selection of PD-1 inhibitors/PD-1 inhibitors combined with chemotherapy as salvage therapy in R/R DLBCL patients after anti-CD19-CAR T-cell therapy
- PMID: 40474425
- PMCID: PMC12141794
- DOI: 10.1177/09636897251338713
An exploration of the initiation time and patient selection of PD-1 inhibitors/PD-1 inhibitors combined with chemotherapy as salvage therapy in R/R DLBCL patients after anti-CD19-CAR T-cell therapy
Abstract
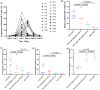
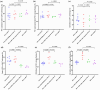
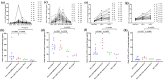
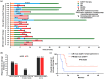
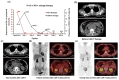
A significant proportion of patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) exhibit no response to chimeric antigen receptor (CAR) T-cell therapy or suffer from disease progression thereafter. This study investigated the efficacy and safety of salvage therapy with PD-1 inhibitor-based combination treatment and the patient selection after their CAR T-cell therapy. Twenty-one patients with R/R DLBCL and a high tumor burden were treated with CAR T-cell therapy, a treatment that has shown promising results in clinical trials and has been approved by the Food and Drug Administration (FDA) for use in DLBCL. Patients who achieved complete response (CR) with the CAR T-cell therapy received salvage therapy when their disease progressed again. Patients who obtained partial response (PR) or stable disease (SD) with the CAR T-cell therapy received salvage therapy immediately. Salvage therapy consisted of single PD-1 inhibitors or PD-1 inhibitors combined with chemotherapy. We observed the overall response rate (ORR), overall survival (OS), CAR T-cell amplification, the expression of PD-1, CD3+ T cells, cytokines, and the adverse events. For instance, in a clinical trial of LCAR-B38M CAR T-cell therapy, an 88% ORR was observed, with 74% of patients achieving CR and a median duration of response (DOR) of 16 months. The ORR and CR of the salvage therapy were 28.57% and 19.05%, respectively. The ORR and CR were 38.46% and 30.77% in the 13 patients who achieved PR/SD with the CAR T-cell therapy and received salvage therapy 2 months after CAR T-cell infusion. But the ORR and CR were only 12.5% and 0%, respectively, in patients who achieved CR with the CAR T-cell therapy and received salvage therapy when they experienced disease re-progression. The ratio of CAR-T cells on day 7/day 14 was lower in the PR in CAR-T (effective to PD-1) group. Before salvage therapy, the percentage of CD3+ T cells was higher in the PR in CAR-T (effective to PD-1) group. There was no difference in the Common Terminology Criteria for Adverse Events (CTCAE) grades among the four groups in the salvage therapy. PD-1 inhibitor-based salvage therapy in patients with R/R DLBCL following the CAR T-cell therapy could be an effective and safe treatment, especially in patients who achieved PR after the CAR T-cell therapy and received this salvage therapy immediately.Trial registration number: ChiCTR1800019622 and ChiCTR1900025310.
Keywords: chimeric antigen receptor (CAR); diffuse large B-cell lymphoma; programmed cell death 1 inhibitors; relapsed/refractory; salvage therapy.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Efficacy of programmed cell death 1 inhibitor maintenance therapy after combined treatment with programmed cell death 1 inhibitors and anti-CD19-chimeric antigen receptor T cells in patients with relapsed/refractory diffuse large B-cell lymphoma and high tumor burden.Hematol Oncol. 2023 Apr;41(2):275-284. doi: 10.1002/hon.2981. Epub 2022 Mar 1. Hematol Oncol. 2023. PMID: 35195933
-
CD19 CAR-T expressing PD-1/CD28 chimeric switch receptor as a salvage therapy for DLBCL patients treated with different CD19-directed CAR T-cell therapies.J Hematol Oncol. 2021 Feb 16;14(1):26. doi: 10.1186/s13045-021-01044-y. J Hematol Oncol. 2021. PMID: 33593414 Free PMC article.
-
Knockout IL4I1 affects macrophages to improve poor efficacy of CD19 CAR-T combined with PD-1 inhibitor in relapsed/refractory diffuse large B-cell lymphoma.J Transl Med. 2025 Jan 22;23(1):105. doi: 10.1186/s12967-024-06028-3. J Transl Med. 2025. PMID: 39844281 Free PMC article.
-
The Improving Outcomes in Relapsed-Refractory Diffuse Large B Cell Lymphoma: The Role of CAR T-Cell Therapy.Curr Treat Options Oncol. 2025 Jun;26(6):445-464. doi: 10.1007/s11864-025-01305-9. Epub 2025 Apr 28. Curr Treat Options Oncol. 2025. PMID: 40293655 Review.
-
Meta-analysis of response rates to first-line salvage treatment after CAR-T therapy failure in large B-cell lymphoma patients.Expert Opin Biol Ther. 2024 May;24(5):389-397. doi: 10.1080/14712598.2024.2354371. Epub 2024 May 16. Expert Opin Biol Ther. 2024. PMID: 38725262 Review.
References
-
- Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I, et al.. JULIET investigators. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45–56. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, Sehgal A, et al.. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52. - PubMed
-
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, Raffeld M, et al.. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–49. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

