A Group 6 LEA Protein Plays Key Roles in Tolerance to Water Deficit, and in Maintaining the Glassy State and Longevity of Seeds
- PMID: 40474454
- PMCID: PMC12319291
- DOI: 10.1111/pce.15649
A Group 6 LEA Protein Plays Key Roles in Tolerance to Water Deficit, and in Maintaining the Glassy State and Longevity of Seeds
Abstract
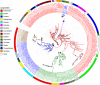
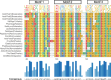
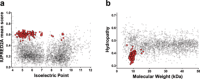
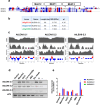
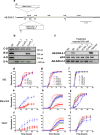
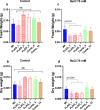
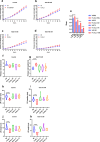
Plants have a wide range of adaptive and protective mechanisms to cope with dehydration. Central in these processes are the Late Embryogenesis Abundant (LEA) proteins, whose levels notably increase in response to dehydration during seed development and vegetative tissues. Understanding the function of LEA proteins is essential for gaining insights into plant development and their adjusting responses to environmental stress. This study focuses on Group 6 LEA proteins (LEA6) from Arabidopsis thaliana: AtLEA6-2.1, AtLEA6-2.2, and AtLEA6-2.3. Phylogenetic analysis reveals that LEA6 family emerged with seed plants, pointing to a unique role in seed viability. Functional characterization using T-DNA insertion mutants demonstrated that AtLEA6-2.1, but not AtLEA6-2.2, is essential for tolerance to high-osmolarity and salinity during germination and post-germination growth. AtLEA6-2.1 deficiency also altered root architecture under salinity, increasing primary root length while reducing lateral root number and length, suggesting a role in root development not described before for a LEA protein. Furthermore, AtLEA6-2.1 is critical for seed longevity, as mutants lacking this protein showed reduced germination after natural and accelerated aging. These mutants exhibited increased glass-former fragility, indicating that AtLEA6-2.1 deficiency reduces cellular viscosity, which we found correlates with reduced longevity. Our investigation extends to protective protein assays under dehydration, revealing that the acidic nature of this protein family requires specific conditions for its In Vitro protective activity. Overall, this study underscores the essential role of AtLEA6-2.1 in the plant response to low-water availability, seed longevity, and glassy state properties, making it a potential target for enhancing plant resilience to environmental challenges.
Keywords: Arabidopsis thaliana; LEA proteins; intrinsically disordered proteins; seed longevity; water deficit.
© 2025 The Author(s). Plant, Cell & Environment published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

