Battery longevity of a helix-fixation dual-chamber leadless pacemaker: results from the AVEIR DR i2i Study
- PMID: 40474610
- PMCID: PMC12141743
- DOI: 10.1093/europace/euaf074
Battery longevity of a helix-fixation dual-chamber leadless pacemaker: results from the AVEIR DR i2i Study
Abstract
Aims: A dual-chamber leadless pacemaker (LP) system that employs distinct atrial and ventricular LP devices (ALP, VLP) has been introduced to clinical practice. Proprietary, low-energy, implant-to-implant (i2i) communication at each beat enables the devices to maintain synchronous atrioventricular sensing and pacing. We evaluated device longevities and contributing factors, such as i2i communication.
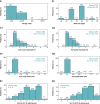
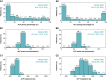
Methods and results: Patients meeting dual-chamber pacing indications received the dual-chamber LP system as part of a prospective, multi-centre, international clinical trial (Aveir DR i2i Study, NCT05252702). Programming and diagnostics were interrogated from all de novo, non-revised, dual-chamber programmed devices at 12 months post-implant. This analysis included 302 patients (65% male; age 70 ± 13 years; weight 80 ± 19 kg; intrinsic heart rate 55 ± 7 bpm; 58% sinus node dysfunction, 27% atrioventricular block). At 12 months, devices were programmed to dual-chamber pacing (DDD(R) or DDI(R)) at a median 60 bpm rate, median 1.25 V pulse amplitude in ALP and 1.5 V in VLP, median 0.4 ms pulse width, and median i2i signal setting level 5 out of 7. Median ALP and VLP remaining battery longevities at 12 months were 4.3 and 9.1 years, with median total ALP and VLP longevities of 5.3 and 9.9 years. Base rate, pulse amplitude, pacing percentage, event rate, impedance, and i2i setting level all exhibited significant correlations with ALP and VLP longevities (P < 0.001). Programming i2i setting levels below 7 produced the greatest longevity savings.
Conclusion: The first dual-chamber LP demonstrated adequate projected battery longevity after 12 months of use. Patient-specific device programming considerations, unique to leadless devices, may extend longevity.
Keywords: Aveir; Battery longevity; Dual-chamber; Leadless pacemaker.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: V.Y.R. is a consultant to Abbott; and unrelated to this manuscript, he serves as a consultant for and has equity in Ablacon, Acutus Medical, Affera-Medtronic, Anumana, Apama Medical-Boston Scientific, APN Health, Append Medical, Aquaheart, Atacor, Autonomix, Axon Therapies, Backbeat, BioSig, CardiaCare, Cardiofocus, CardioNXT/AFTx, Circa Scientific, CoRISMA, Corvia Medical, Dinova-Hangzhou DiNovA EP Technology, East End Medical, EPD-Philips, EP Frontiers, Epix Therapeutics-Medtronic, EpiEP, Eximo, Farapulse-Boston Scientific, Field Medical, Focused Therapeutics, HRT, Intershunt, Javelin, Kardium, Keystone Heart, Laminar Medical, LuxMed, Medlumics, Middlepeak, Neutrace, Nuvera-Biosense Webster, Oracle Health, Pulse Biosciences, Restore Medical, Sirona Medical, SoundCath, and Valcare; and unrelated to this work, he has served as a consultant for Adagio Medical, AtriAN, Biosense-Webster, BioTel Heart, Biotronik, Boston Scientific, Cairdac, Cardionomic, CoreMap, Fire1, Gore & Associates, Impulse Dynamics, Medtronic, Novartis, Novo Nordisk, Philips; and unrelated to this work, he has equity in Atraverse, DRS Vascular, Manual Surgical Sciences, Newpace, Nyra Medical, Soundcath, Surecor, and Vizaramed. R.D. reports consulting fees from Abbott and serves as a steering committee member for Abbott. J.E.I. reports consulting fees from Abbott and Medtronic, serves as a steering committee member of Abbott and a data safety monitoring committee member of Boston Scientific. P.D. reports research grants from Abbott, Boston Scientific, and Medtronic. D.V.E. reports consulting fees and research grants from Abbott and Medtronic; and unrelated to this manuscript reports consulting fees and research grants from Boston Scientific and GE Healthcare, as well equity in CorVista, Clarius, eMurmur, HelpWear, and ProtonIntel. R.C. reports consulting fees from Medtronic. M.S. serves as a steering committee member for Abbott. M.G.B. serves as a steering committee member for Abbott. G.H. serves as a steering committee member for Abbott. T.C. reports consulting fees and honoraria from Abbott. N.B. and M.G.F. are employees of Abbott. R.E.K. reports research grants, consulting fees, and serves as a steering committee member of Abbott and Boston Scientific; and reports speaking fees from Medtronic.
Figures




Comment in
-
Getting the most out of dual-chamber leadless pacing: how to prolong battery longevity?Europace. 2025 Jul 1;27(7):euaf073. doi: 10.1093/europace/euaf073. Europace. 2025. PMID: 40601298 Free PMC article.
References
-
- Reddy VY, Exner DV, Doshi R, Tomassoni G, Bunch TJ, Estes NAM, et al. Primary results on safety and efficacy from the LEADLESS II-Phase 2 worldwide clinical trial. JACC Clin Electrophysiol 2022;8:115–7. - PubMed
-
- Cantillon DJ, Dukkipati SR, Ip JH, Exner DV, Niazi IK, Banker RS, et al. Comparative study of acute and mid-term complications with leadless and transvenous cardiac pacemakers. Heart Rhythm 2018;15:1023–30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

