Fungal β-glucan instructed miR-32-5p modulates Dectin-1 signaling mediated inflammation, reactive oxygen species and apoptosis through polarization of "M2a-like" macrophage in Candida colitis
- PMID: 40474676
- PMCID: PMC12147485
- DOI: 10.1080/21505594.2025.2514789
Fungal β-glucan instructed miR-32-5p modulates Dectin-1 signaling mediated inflammation, reactive oxygen species and apoptosis through polarization of "M2a-like" macrophage in Candida colitis
Abstract
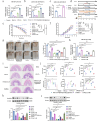
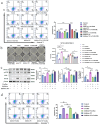
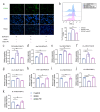
Ulcerative colitis (UC) is a chronic and easy-to-relapse intestinal disease characterized by colon inflammation and microbial dysbiosis. Candida albicans is the most common fungal resident in the human gut. Overgrowth of C. albicans has been linked to the aggravation of UC. Previously, we demonstrated that miR-32-5p was the most differentially expressed microRNA in DSS-induced colitis model supplemented with C. albicans and might be a potential target in the treatment of Candida colitis. However, the underlying pathogenic and therapeutic mechanisms remain unclear. Here, we firstly used the ITS technique to analyse intestinal mycobiota. The miR-32-5p adenovirus in company with extracted Candida cell wall β-glucan were then employed to monitor the impacts of miR-32-5p and fungal β-glucan on the severity of Candida colitis. Subsequently, gene silencing together with inhibitors of reactive oxygen species (ROS) and apoptosis was used to survey the modulation of miR-32-5p on macrophage polarization. According to these results, C. albicans became the dominant intestinal fungal species in Candida colitis model. Candida β-glucan could instruct miR-32-5p expression to affect the severity of Candida colitis in a concentration-dependent manner. Interestingly, high fungal β-glucan with pro-inflammatory effects hindered further increases in gut inflammation. Further analysis showed that overexpression of miR-32-5p could effectively inhibit inflammation and apoptosis and enhance phagocytosis and ROS production through Dectin-1 signalling in macrophages. A panel of representative gene expressions verified the polarization of the M2-like phenotype induced by miR-32-5p. Mechanistically, our results reveal the therapeutic potential of miR-32-5p in the amelioration of Candida colitis.
Keywords: Candida albicans; Dectin-1; Ulcerative colitis; miR-32-5p; oxidative stress; β-glucan.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
-
- Zwolinska-Wcislo M, Brzozowski T, Budak A, et al. Effect of candida colonization on human ulcerative colitis and the healing of inflammatory changes of the colon in the experimental model of colitis ulcerosa. J Physiol Pharmacol. 2009;60(1):107–118. - PubMed
-
- Catalán-Serra I, Thorsvik S, Beisvag V, et al. Fungal microbiota composition in inflammatory bowel disease patients: characterization in different phenotypes and correlation with clinical activity and disease course. Inflamm Bowel Dis. 2024;30(7):1164–1177. doi: 10.1093/ibd/izad289 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
