Influence of Cholesterol on the Insertion and Interaction of SARS-CoV-2 Proteins with Lipid Membranes
- PMID: 40474679
- PMCID: PMC12175161
- DOI: 10.1021/acsabm.5c00776
Influence of Cholesterol on the Insertion and Interaction of SARS-CoV-2 Proteins with Lipid Membranes
Abstract
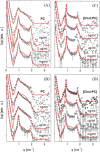
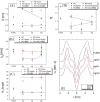
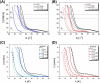
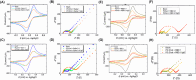
Cholesterol is an essential sterol in cell membranes that regulates organization and fluidity. This biomolecule has been identified as one of the critical factors in the internalization process of various viruses in human cells. Therefore, understanding these mechanisms is crucial for a deeper comprehension of viral pathogenicity in the search for practical therapeutic approaches against viral diseases. The biochemical and biophysical processes related to these diseases are highly complex. For this reason, studying model systems capable of mimicking the interaction of lipid membranes with cholesterol and proteins is fundamental. In this work, we propose to study the structural and elastic changes in mono-, bi-, and tridimensional lipid systems composed of dipalmitoylphosphatidylcholine (PC) with varying amounts of cholesterol in the presence and absence of the S protein (Spike) and its receptor-binding domain (RBD) from SARS-CoV-2. To characterize these systems, we used both experimental and theoretical approaches such as Langmuir trough, atomic force microscopy (AFM), small-angle X-ray scattering (SAXS), electrochemical methods, and molecular dynamics (MD) simulations. With the interpretation of all results obtained in this work, it was possible to propose a structural model of the membrane in the presence of cholesterol and the interaction with the Spike protein and RBD. The behavior of the adsorption isotherm and SAXS data, together with the results provided by MD simulations, led us to conclude that cholesterol in PC monolayers promotes local alterations, inducing the formation of more rigid membrane regions. More importantly, cholesterol plays a crucial role in facilitating the allocation of SARS-CoV-2 proteins in lipid systems. This is especially true for the Spike protein, which displayed a non-ACE2 mediated stable binding to the lipid membrane with high internalization.
Keywords: Langmuir monolayer; SARS-CoV-2; atomic force microscopy; cholesterol dynamics; dipalmitoylphosphatidylcholine; lipid membranes; molecular dynamics simulations; small angle X-ray scattering.
Figures


Similar articles
-
Determinants of susceptibility to SARS-CoV-2 infection in murine ACE2.J Virol. 2025 Jun 17;99(6):e0054325. doi: 10.1128/jvi.00543-25. Epub 2025 May 12. J Virol. 2025. PMID: 40353671 Free PMC article.
-
Comprehensive Insights into the Cholesterol-Mediated Modulation of Membrane Function Through Molecular Dynamics Simulations.Membranes (Basel). 2025 Jun 8;15(6):173. doi: 10.3390/membranes15060173. Membranes (Basel). 2025. PMID: 40559352 Free PMC article. Review.
-
Conformational Dynamics and Binding Interactions of SARS-CoV-2 Spike Protein Variants: Omicron, XBB.1.9.2, and EG.5.J Chem Inf Model. 2025 Jul 28;65(14):7651-7667. doi: 10.1021/acs.jcim.5c00308. Epub 2025 Jul 11. J Chem Inf Model. 2025. PMID: 40643983 Free PMC article.
-
Pulling Forces Differentially Affect Refolding Pathways Due to Entangled Misfolded States in SARS-CoV-1 and SARS-CoV-2 Receptor Binding Domain.Biomolecules. 2024 Oct 18;14(10):1327. doi: 10.3390/biom14101327. Biomolecules. 2024. PMID: 39456260 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
